Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 July 2017
NewsHuman
Eleven medicines recommended for approval, including five orphans
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended eleven medicines for approval at its July meeting.
The CHMP recommended granting a marketing authorisation for Verkazia (ciclosporin), an orphan medicine to treat severe vernal keratoconjunctivitis in children and adolescents. This medicine was reviewed under EMA's accelerated assessment mechanism. For more information, please see the press release in the grid below.
Four other orphan medicines were recommended for approval by the Committee: Bavencio (avelumab) for the treatment of Merkel cell carcinoma; Lutathera (lutetium [177Lu] oxodotreotide), for the treatment of gastro-entero-pancreatic neuroendocrine tumours; Rydapt (midostaurin), for the treatment of acute myeloid leukaemia, aggressive systemic mastocytosis, systemic mastocytosis with associated haematological neoplasm and mast cell leukaemia; and Xermelo (telotristat ethyl), for the treatment of carcinoid syndrome.
Dupixent (dupilumab) received a positive opinion for the treatment of atopic dermatitis.
The CHMP adopted a positive opinion for Symtuza (darunavir / cobicistat / emtricitabine / tenofovir alafenamide) for the treatment of HIV infection.
Tecentriq (atezolizumab) received a positive opinion for the treatment of locally advanced or metastatic urothelial carcinoma and of non-small cell lung cancer.
Three generic medicines received a positive opinion from the CHMP: Entecavir Accord (entecavir) and Entecavir Mylan (entecavir), both for the treatment of chronic hepatitis B; and Lacosamide Accord (lacosamide), for the treatment of epilepsy.
Negative opinions on two new medicines
The CHMP adopted a negative opinion for Fanaptum (iloperidone). Fanaptum was expected to be used to treat schizophrenia.
The Committee also adopted a negative opinion for Onzeald (etirinotecan pegol). Onzeald was intended to be used to treat breast cancer with brain metastases.
For more information on these negative opinions, please see the questions-and-answers documents in the grid below.
Eight recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Bydureon, Gazyvaro, Humira, Keytruda, RoActemra, Signifor, Sovaldi and Vimpat.
Outcome of review on gadolinium contrast agents
The CHMP recommended restricting the use of some linear gadolinium agents used in magnetic resonance imaging (MRI) body scans and suspending the authorisations of others. For more information please see the public health communication in the grid below.
Re-examination of arbitration procedure
The applicant for Alcover 750 mg, 1250 mg, 1750 mg granules (sodium oxybate) has requested a re-examination of the CHMP's June 2017 opinion. Upon receipt of the grounds for the request, the CHMP will re-examine its opinion and issue a final recommendation.
Withdrawal of application
The application for an initial marketing authorisation for Infinia (alpha-1-antitrypsin) was withdrawn. This medicine was intended to be used for the treatment of adults with lung disease due to congenital deficiency of alpha-1-antitrypsin. A questions-and-answers document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the July 2017 meeting is published on EMA's website. Minutes of the June 2017 CHMP meeting will be published in the coming weeks.
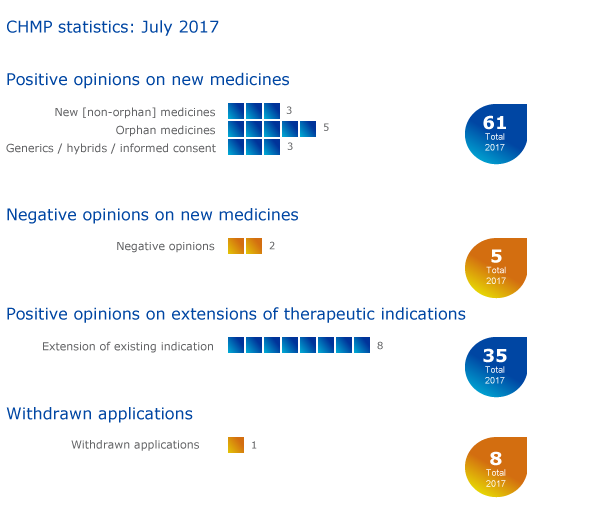
CHMP statistics
Key figures from the July 2017 CHMP meeting are represented in the graphic below.
| Name of medicine | Bavencio |
|---|---|
| International non-proprietary name (INN) | avelumab |
| Marketing-authorisation applicant | Merck Serono Europe Ltd |
| Therapeutic indication |
Treatment of metastatic Merkel cell carcinoma |
| More information | CHMP summary of positive opinion for Bavencio |
| Name of medicine | Dupixent |
|---|---|
| INN | dupilumab |
| Marketing-authorisation applicant | Sanofi-Aventis groupe |
| Therapeutic indication | Treatment of atopic dermatitis |
| More information | CHMP summary of positive opinion for Dupixent |
| Name of medicine | Lutathera |
|---|---|
| INN | lutetium (177lu) oxodotreotide |
| Marketing-authorisation applicant | Advanced Accelerator Applications |
| Therapeutic indication | Treatment of well differentiated gastroenteropancreatic neuroendocrine tumours |
| More information | CHMP summary of positive opinion for Lutathera |
| Name of medicine | Rydapt |
|---|---|
| INN | midostaurin |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| Therapeutic indication | Treatment of acute myeloid leukaemia, aggressive systemic mastocytosis, systemic mastocytosis with associated haematological neoplasm and mast cell leukaemia |
| More information | CHMP summary of positive opinion for Rydapt |
| Name of medicine | Symtuza |
|---|---|
| INN | darunavir / cobicistat / emtricitabine / tenofovir alafenamide |
| Marketing-authorisation applicant | Janssen-Cilag International N.V. |
| Therapeutic indication | Treatment of HIV infection |
| More information | CHMP summary of positive opinion for Symtuza |
| Name of medicine | Tecentriq |
|---|---|
| INN | atezolizumab |
| Marketing-authorisation applicant | Roche Registration Ltd |
| Therapeutic indication | Treatment of locally advanced or metastatic urothelial carcinoma and of non-small cell lung cancer |
| More information | CHMP summary of positive opinion for Tecentriq |
| Name of medicine | Verkazia |
|---|---|
| INN | ciclosporin |
| Marketing-authorisation applicant | Santen Oy |
| Therapeutic indication | Treatment of severe vernal keratoconjunctivitis |
| More information | CHMP summary of positive opinion for Verkazia
Press release: New medicine for rare form of eye allergy in children and teenagers |
| Name of medicine | Xermelo |
|---|---|
| INN | telotristat ethyl |
| Marketing-authorisation applicant | Ipsen Pharma |
| Therapeutic indication |
Treatment of carcinoid syndrome diarrhoea in combination with a somatostatin analogue |
| More information | CHMP summary of positive opinion for Xermelo |
| Name of medicine | Entecavir Accord |
|---|---|
| INN | entecavir |
| Marketing-authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of chronic hepatitis B |
| More information | CHMP summary of positive opinion for Entecavir Accord |
| Name of medicine | Entecavir Mylan |
|---|---|
| INN | entecavir |
| Marketing-authorisation applicant | Mylan S.A.S. |
| Therapeutic indication | Treatment of chronic hepatitis B |
| More information | CHMP summary of positive opinion for Entecavir Mylan |
| Name of medicine | Lacosamide Accord |
|---|---|
| INN | lacosamide |
| Marketing-authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of epilepsy |
| More information | CHMP summary of positive opinion for Lacosamide Accord |
| Name of medicine | Fanaptum |
|---|---|
| INN | iloperidone |
| Marketing-authorisation applicant | Vanda Pharmaceuticals Ltd |
| Therapeutic indication | Treatment of schizophrenia |
| More information | Questions and answers on the refusal of the marketing authorisation for Fanaptum (iloperidone) |
| Name of medicine | Onzeald |
|---|---|
| INN | etirinotecan pegol |
| Marketing-authorisation applicant | Nektar Therapeutics UK Ltd |
| Therapeutic indication | Treatment of advanced breast cancer which has spread to the brain |
| More information | Questions and answers on the refusal of the marketing authorisation for Onzeald (etirinotecan pegol) |
| Name of medicine | Bydureon |
|---|---|
| INN | exenatide |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | CHMP post-authorisation summary of positive opinion for Bydureon |
| Name of medicine | Gazyvaro |
|---|---|
| INN | obinutuzumab |
| Marketing-authorisation holder | Roche Registration Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Gazyvaro (II-16) |
| Name of medicine | Humira |
|---|---|
| INN | adalimumab |
| Marketing-authorisation holder | AbbVie Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Humira (II-163) |
| Name of medicine | Keytruda |
|---|---|
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Keytruda |
| Name of medicine | RoActemra |
|---|---|
| INN | tocilizumab |
| Marketing-authorisation holder | Roche Registration Ltd |
| More information | CHMP post-authorisation summary of positive opinion for RoActemra (II-66) |
| Name of medicine | Signifor |
|---|---|
| INN | pasireotide |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Signifor (X-30-G) |
| Name of medicine | Sovaldi |
|---|---|
| INN | sofosbuvir |
| Marketing-authorisation holder | Gilead Sciences International Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Sovaldi |
| Name of medicine | Vimpat |
|---|---|
| INN | lacosamide |
| Marketing-authorisation holder | UCB Pharma S.A. |
| More information | CHMP post-authorisation summary of positive opinion for Vimpat (II-65-G) |
| Name of medicine | Gadolinium-containing contrast agents |
|---|---|
| INN | gadobenic acid / gadobutrol / gadodiamide / gadopentetic acid / gadoteric acid / gadoteridol / gadoversetamide / gadoxetic acid |
| More information | EMA's final opinion confirms restrictions on use of linear gadolinium agents in body scans |
| Name of medicine | Alcover 750 mg, 1250 mg, 1750 mg granules |
|---|---|
| INN | sodium oxybate |
| Marketing-authorisation holder | D&A Pharma |
| More information | Questions and answers on Alcover granules (sodium oxybate 750, 1250 and 1750 mg) |
| Name of medicine | Infinia |
|---|---|
| INN | alpha-1-antitrypsin |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Infinia (alpha-1-antitrypsin) |
| Recommendations on eligibility to PRIME scheme - Adopted at the CHMP meeting of 17-20 July 2017 |
| Overview of (invented) names reviewed in June 2017 by the Name Review Group (NRG) |
| Scientific advice and protocol assistance adopted during the CHMP meeting 17 – 20 July 2017 |
| Start of Community reviews |