
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 March 2024
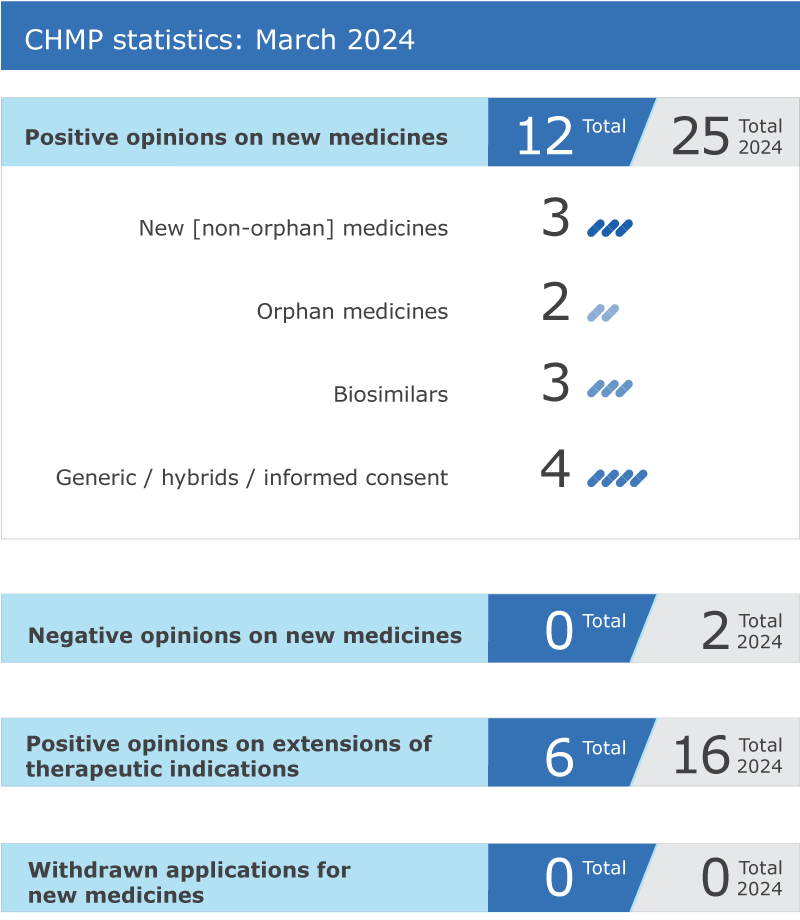
EMA’s human medicines committee (CHMP) recommended 12 medicines for approval at its March 2024 meeting.
NewsHumanMedicinesReferrals
EMA’s human medicines committee (CHMP) recommended 12 medicines for approval at its March 2024 meeting.
The CHMP recommended granting a marketing authorisation for Awiqli (insulin icodec) for the treatment of diabetes mellitus in adults.
The committee adopted a positive opinion for Emblaveo (aztreonam-avibactam), an antibiotic indicated for the treatment of complicated intra-abdominal and urinary tract infections, hospital-acquired pneumonia and infections caused by certain types of bacteria (aerobic Gram-negative) that are resistant to many currently available antibiotics and where patients have limited or sometimes no treatment options. Emblaveo was evaluated under EMA's accelerated assessment mechanism because it is considered to address an unmet medical need. See more details in the news announcement in the grid below.
The CHMP gave a positive opinion for Fabhalta* (iptacopan), an oral treatment for adults with paroxysmal nocturnal haemoglobinuria, a rare genetic disorder and potentially life-threatening blood disease leading to the premature destruction of red blood cells by the immune system. This medicine was supported through EMA’s Priority Medicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support for promising medicines with a potential to address unmet medical needs. See more details in the news announcement in the grid below.
Lytenava (bevacizumab) received a positive opinion from the CHMP for the treatment of neovascular age-related macular degeneration, a progressive retinal macular disease causing gradual vision impairment mainly in the elderly.
The committee adopted positive opinions for three biosimilar medicines:
The CHMP recommended granting a marketing authorisation for Agilus* (dantrolene sodium, hemiheptahydrate), indicated in adults and children of all ages for the treatment of malignant hyperthermia, a life threatening emergency condition in which the skeletal muscles of the body are over-stimulated and are unable to relax. This can cause a very fast increase of body temperature and/or a build-up of waste products in the body (metabolic acidosis), which can stop vital organs from working properly. A paediatric-use marketing authorisation (PUMA) was recommended for Neoatricon (dopamine hydrochloride), for the treatment of hypotension in neonates, infants and children. Both medicines were submitted in hybrid applications, which rely in part on the results of pre-clinical tests and clinical trials of an already authorised reference product and in part on new data.
Three generic medicines also received a positive opinion from the committee: Dimethyl fumarate Accord (dimethyl fumarate), Dimethyl fumarate Mylan (dimethyl fumarate) and Dimethyl fumarate Neuraxpharm (dimethyl fumarate). All three medicines are indicated for the treatment of adult and paediatric patients aged 13 years and older with relapsing remitting multiple sclerosis, a disease of the brain and spinal cord in which inflammation destroys the protective covering around nerves and the nerves themselves.
The committee recommended extensions of indication for six medicines that are already authorised in the European Union (EU): Bimzelx, Nilemdo, Nustendi, Onivyde pegylated liposomal*, Retsevmo and Xtandi.
The applications to extend the therapeutic indications of four medicines were withdrawn:
Question-and-answer documents on these withdrawals to extend therapeutic indications are available in the grid below.
The CHMP completed a review of Micrazym, a pancreatic enzyme replacement therapy, following a disagreement among EU Member States regarding its authorisation via national procedures. The committee concluded that the benefits of Micrazym outweigh its risks, and the marketing authorisation should be granted in the Netherlands and in the Member States of the EU where the company has applied for a marketing authorisation.
For more information, see the Q&A document in the grid below.
The CHMP confirmed its recommendation to suspend or not grant the marketing authorisations of a number of generic medicines tested by Synapse Labs Pvt. Ltd, a contract research organisation located in Pune, India. This confirmation concludes the re-examination requested by the applicants and marketing authorisation holders for some of the medicines concerned.
For more information, see public health communication in the grid below.
The CHMP was updated on the outcome of the appellate judgment of the Court of Justice of 14 March 2024 in Case C-291/22 P. The judgment examined questions related to the organisation of EMA’s Scientific Advisory Groups (SAGs). SAGs are groups of scientific experts that are called upon to respond to specific questions posed by EMA’s committees during the evaluation of a medicine. In particular, the judgment has implications on EMA’s policy on the handling of competing interests of experts, in relation to SAG members. EMA is currently in the process of carefully considering any needed revisions of its policy in relation to SAGs. Any possible revisions of EMA’s policy will be communicated in due time.
The agenda of the March 2024 CHMP meeting is published on EMA's website. Minutes of the meeting will be published in the coming weeks.
Key figures from the March 2024 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
insulin icodec
Novo Nordisk A/S
Treatment of diabetes mellitus in adults.
aztreonam / avibactam
Pfizer Europe Ma EEIG
Treatment of complicated Intra-Abdominal Infection (cIAI), complicated Urinary Tract Infection (cUTI), including pyelonephritis, Hospital-acquired pneumonia (HAP), including ventilator associated pneumonia (VAP), and aerobic Gram-negative infections with limited treatment options.
iptacopan
Novartis Europharm Limited
Treatment of paroxysmal nocturnal haemoglobinuria.
denosumab
Sandoz GmbH
Treatment of osteoporosis.
omalizumab
Celltrion Healthcare Hungary Kft.
Treatment of asthma.
denosumab
Sandoz GmbH
Prevention of skeletal related events with advanced malignancies.
dantrolene sodium, hemiheptahydrate
Norgine B.V.
In combination with adequate support measures, Agilus is indicated for the treatment of malignant hyperthermia in adults and children of all ages.
dopamine hydrochloride
BrePco Biopharma Limited
Treatment of hypotension in neonates, infants and children.
dimethyl fumarate
Accord Healthcare
For the treatment of adult and paediatric patients aged 13 years and older with relapsing remitting multiple sclerosis (RRMS).
dimethyl fumarate
Mylan Ireland Limited
For the treatment of adult and paediatric patients aged 13 years and older with relapsing remitting multiple sclerosis (RRMS).
dimethyl fumarate
Neuraxpharm Pharmaceuticals S.L.
For the treatment of adult and paediatric patients aged 13 years and older with relapsing remitting multiple sclerosis (RRMS).
bimekizumab
UCB Pharma S.A
bempedoic acid
Daiichi Sankyo Europe GmbH
bempedoic acid / ezetimibe
Daiichi Sankyo Europe GmbH
irinotecan hydrochloride trihydrate
Les Laboratoires Servier
selpercatinib
Eli Lilly Nederland B.V.
enzalutamide
Astellas Pharma Europe B.V.
brentuximab vedotin
Takeda Pharma A/S
opicapone
Bial Portela & Companhia S.A.
Ongentys: Withdrawn application
opicapone
Bial Portela & Companhia S.A.
Ontilyv: Withdrawn application
abatacept
Bristol-Myers Squibb Pharma
porcine pancreas enzymes
Avva Pharmaceuticals Ltd.