Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 May 2015
NewsHuman
Eight new medicines, including three cancer immunotherapies, recommended for approval
Eight new medicines have been recommended for approval at the May 2015 meeting of the Committee for Medicinal Products for Human Use (CHMP).
The Committee has recommended granting a marketing authorisation for Repatha (evolocumab), a first-in-class treatment to lower high levels of cholesterol in the blood of people who are unable to control their cholesterol despite taking optimal doses of statins or who cannot take statins. Repatha is also recommended to treat homozygous familial hypercholesterolaemia, a rare inherited disorder. Repatha is the first monoclonal antibody in this therapeutic area and provides a new treatment option for patients who are unable to control their high cholesterol despite taking currently available therapies. For more information on Repatha, please see the press release in the grid below.
Three immunotherapies for the treatment of different types of cancer were granted a positive opinion by the CHMP: Keytruda (pembrolizumab) to treat advanced (unresectable or metastatic) melanoma, Nivolumab BMS (nivolumab) to treat adults with squamous non-small cell lung cancer (NSCLC) and Unituxin (dinutiximab) for the treatment of high-risk neuroblastoma - a type of cancer that most often occurs in young children. Unituxin was granted an orphan designation in 2011. Cancer immunotherapies are treatments that use the body's own immune system to fight the disease. For more information on these cancer medicines, please see the press releases in the grid below.
The CHMP recommended Evotaz (atazanavir / cobicistat) intended for the treatment of HIV-1 infected adults without known mutations associated with resistance to atazanavir.
Omidria (phenylephrine / ketorolac) received a positive opinion from the Committee for maintenance of intraoperative mydriasis, prevention of intraoperative miosis and reduction of acute postoperative ocular pain in intraocular lens replacement surgery.
Two generic medicines received positive opinions from the CHMP: Bortezomib Accord (bortezomib) for the treatment of multiple myeloma and mantle cell lymphoma and Pregabalin Zentiva (pregabalin) for the treatment of epilepsy and generalised anxiety disorder.
Six recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Fycompa, Imbruvica, Kuvan, Simponi, Stelara and Xultophy. For more information on the extension of indication for Imbruvica, please see the press release in the grid below.
Outcome of re-examination of GVK Bio Sciences
The CHMP has confirmed its recommendation to suspend a number of medicines for which authorisation in the European Union were primarily based on clinical studies conducted at GVK Biosciences in Hyderabad, India. This is the outcome of a re-examination requested by marketing authorisation holders for seven of the medicines concerned.
Withdrawal of applications
The applications for marketing authorisation for Aripiprazole Mylan (aripiprazole) and Corluxin (mifepristone) have been withdrawn.
Question-and-answer documents on these withdrawals are available in the grid below.
Agenda and minutes
The agenda of the May 2015 meeting is published on EMA's website. The minutes of the meeting will be published during the week following the June CHMP meeting.
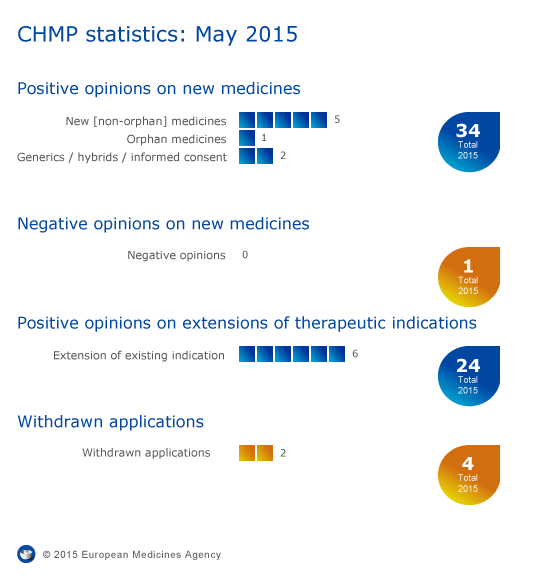
CHMP statistics
Key figures from the May 2015 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's May 2015 meeting, is available in the grid below.
| Name of medicine | Evotaz |
|---|---|
| International non-proprietary name (INN) | atazanavir / cobicistat |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Therapeutic indication | Treatment of HIV-1 infected adults without known mutations associated with resistance to atazanavir |
| More information | CHMP summary of positive opinion for Evotaz |
| Name of medicine | Keytruda |
|---|---|
| INN | pembrolizumab |
| Marketing-authorisation applicant | Merck Sharp & Dohme Limited |
| Therapeutic indication | Treatment of melanoma |
| More information |
CHMP summary of positive opinion for Keytruda
Press release: New treatment option recommended for patients with advanced melanoma |
| Name of medicine | Nivolumab BMS |
|---|---|
| INN | nivolumab |
| Marketing-authorisation applicant | Bristol-Myers Squibb Pharma EEIG |
| Therapeutic indication | Treatment of adults with squamous non-small cell lung cancer (NSCLC) |
| More information |
CHMP summary of positive opinion for Nivolumab BMS
Press release: New treatment option for patients with advanced lung cancer |
| Name of medicine | Omidria |
|---|---|
| INN | phenylephrine / ketorolac |
| Marketing-authorisation applicant | Omeros London Limited |
| Therapeutic indication | Maintenance of intraoperative mydriasis, prevention of intraoperative miosis and reduction of acute postoperative ocular pain in intraocular lens replacement surgery in adults |
| More information | CHMP summary of positive opinion for Omidria |
| Name of medicine | Repatha |
|---|---|
| INN | evolocumab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Therapeutic indication | Hypercholesterolaemia, mixed dyslipidaemia and homozygous familial hypercholesterolaemia |
| More information |
CHMP summary of positive opinion for Repatha
Press release: First-in-class treatment to lower cholesterol |
| Name of medicine | Unituxin |
|---|---|
| INN | dinutuximab |
| Marketing-authorisation applicant | United Therapeutics Europe Ltd |
| Therapeutic indication | Treatment of high-risk neuroblastoma |
| More information |
CHMP summary of positive opinion for Unituxin
Press release: EMA recommends treatment for rare cancer in children |
| Name of medicine | Bortezomib Accord |
|---|---|
| INN | bortezomib |
| Marketing-authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of multiple myeloma |
| More information | CHMP summary of positive opinion for Bortezomib Accord |
| Name of medicine | Pregabalin Zentiva |
|---|---|
| INN | pregabalin |
| Marketing-authorisation applicant | Zentiva, k.s. |
| Therapeutic indication | Treatment of epilepsy and generalised anxiety disorder |
| More information | CHMP summary of positive opinion for Pregabalin Zentiva |
| Name of medicine | Fycompa |
|---|---|
| INN | perampanel |
| Marketing-authorisation holder | Eisai Europe Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Fycompa |
| Name of medicine | Imbruvica |
|---|---|
| INN | ibrutinib |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information |
CHMP post-authorisation summary of positive opinion for Imbruvica
Press release: First medicine for rare blood cancer |
| Name of medicine | Kuvan |
|---|---|
| INN | sapropterin |
| Marketing-authorisation holder | Merck Serono Europe Limited |
| More information | CHMP post-authorisation summary of positive opinion for Kuvan |
| Name of medicine | Simponi |
|---|---|
| INN | golimumab |
| Marketing-authorisation holder | Janssen Biologics B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Simponi |
| Name of medicine | Stelara |
|---|---|
| INN | ustekinumab |
| Marketing-authorisation holder | Janssen-Cilag International N.V. |
| More information | CHMP post-authorisation summary of positive opinion for Stelara |
| Name of medicine | Xultophy |
|---|---|
| INN | insulin degludec / liraglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Xultophy |
| Name of medicine | GVK Biosciences |
|---|---|
| More information | GVK Biosciences: European Medicines Agency confirms recommendation to suspend medicines over flawed studies |
| Name of medicine | Aripiprazole Mylan |
|---|---|
| INN | aripiprazole |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Aripiprazole Mylan (aripiprazole) |
| Name of medicine | Corluxin |
|---|---|
| INN | mifepristone |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Corluxin (mifepristone) |