Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 19-22 June 2017
NewsHuman
Eight medicines recommended for approval, including two medicines for chronic hepatitis C virus (HCV) infection
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended eight new medicines for approval at its June meeting.
The CHMP recommended granting marketing authorisations for Maviret (glecaprevir / pibrentasvir) and Vosevi (sofosbuvir / velpatasvir / voxilaprevir) for the treatment of chronic hepatitis C virus (HCV) infection. Both medicines were reviewed under the EU's accelerated assessment mechanism. For more information on these medicines, please see the joint press release in the grid below.
Two cancer medicines received a positive opinion from the CHMP: Kisqali (ribociclib) for the treatment of locally advanced or metastatic breast cancer and Fotivda (tivozanib) for the treatment of advanced renal cell carcinoma.
Mavenclad (cladribine) received a positive opinion for the treatment of relapsing forms of multiple sclerosis.
One biosimilar medicine was recommended for approval by the Committee: Imraldi (adalimumab) for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, paediatric plaque psoriasis, hidradenitis suppurativa, Crohn's disease, paediatric Crohn's disease, ulcerative colitis and uveitis. A biosimilar medicine is a biological medicine that is highly similar to another biological medicine that is already authorised for use.
Two generic medicines received a positive opinion from the CHMP: Efavirenz / Emtricitabine / Tenofovir disoproxil Mylan (efavirenz / emtricitabine / tenofovir disoproxil) for the treatment of HIV infection and Nitisinone MendeliKABS (nitisinone) for the treatment of hereditary tyrosinemia type 1.
Request for re-examination of three CHMP recommendations
The applicants for Adlumiz (anamorelin hydrochloride), Human IGG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech (human IgG1 monoclonal antibody specific for human interleukin-1 alpha) and Masipro (masitinib) have requested re-examinations of the Committee's negative opinions for these medicines adopted at the May 2017 meeting. The CHMP will now re-examine these opinions and issue final opinions.
For more information on these negative opinions, please see the questions-and-answers documents in the grid below.
Nine recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Faslodex, Harvoni, Kaletra, Mimpara, Orencia, Soliris, SonoVue, Stivarga and Victoza.
Withdrawals of applications
Applications for initial marketing authorisations for Elmisol (levamisole) and Zafiride (NGR-human tumour necrosis factor alpha) have been withdrawn.
Elmisol was intended to be used to treat nephrotic syndrome, a kidney disease. Zafiride was to treat patients with advanced malignant pleural mesothelioma, a cancer of the lining of the lungs usually caused by exposure to asbestos.
Questions-and-answers documents on these withdrawals are available in the grid below.
Outcome of review on Symbioflor 2
The CHMP has concluded that Symbioflor 2 can continue to be used for the treatment of irritable bowel syndrome in adults. However, the medicine should no longer be used more widely to treat functional gastrointestinal disorders, a group of disorders with a variety of causes that may require different treatment approaches. For more information, please see the public health communication in the grid below.
Agenda and minutes
The agenda of the June 2017 meeting is published on EMA's website. Minutes of the May 2017 CHMP meeting will be published in the coming weeks.
CHMP statistics
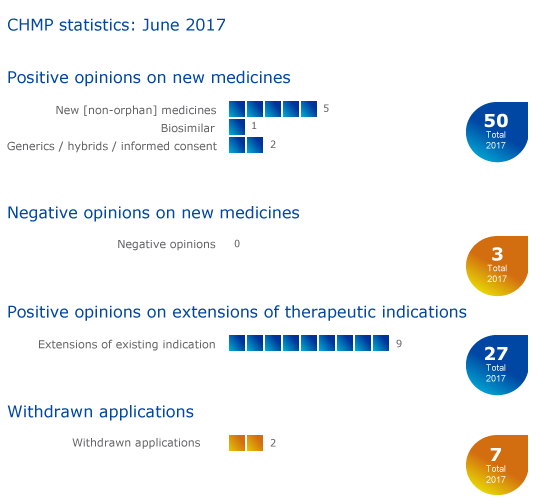
Key figures from the June 2017 CHMP meeting are represented in the graphic below.
| Name of medicine | Fotivda |
|---|---|
| INN | tivozanib |
| Marketing-authorisation applicant | Eusa Pharma |
| Therapeutic indication | Treatment of adult patients with advanced renal cell carcinoma |
| More information | CHMP summary of positive opinion for Fotivda |
| Name of medicine | Kisqali |
|---|---|
| INN | ribociclib |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| Therapeutic indication | Treatment of breast cancer |
| More information | CHMP summary of positive opinion for Kisqali |
| Name of medicine | Mavenclad |
|---|---|
| INN | cladribine |
| Marketing-authorisation applicant | Merck Serono Europe Limited |
| Therapeutic indication | Treatment of relapsing forms of multiple sclerosis |
| More information | CHMP summary of positive opinion for Mavenclad |
| Name of medicine | Maviret |
|---|---|
| INN | glecaprevir / pibrentasvir |
| Marketing-authorisation applicant | AbbVie Ltd |
| Therapeutic indication | Treatment of chronic hepatitis C virus (HCV) infection |
| More information |
CHMP summary of positive opinion for Maviret
Press release: Two new medicines recommended for the treatment of chronic hepatitis C |
| Name of medicine | Vosevi |
|---|---|
| INN | sofosbuvir / velpatasvir / voxilaprevir |
| Marketing-authorisation applicant | Gilead Sciences International Ltd |
| Therapeutic indication | Treatment of chronic hepatitis C virus (HCV) infection |
| More information |
CHMP summary of positive opinion for Vosevi
Press release: Two new medicines recommended for the treatment of chronic hepatitis C |
| Name of medicine | Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan |
|---|---|
| INN | efavirenz / emtricitabine / tenofovir disoproxil |
| Marketing-authorisation applicant | Mylan S.A.S |
| Therapeutic indication | Treatment of HIV infection |
| More information | CHMP summary of positive opinion for Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan |
| Name of medicine | Nitisinone MendeliKABS |
|---|---|
| INN | nitisinone |
| Marketing-authorisation applicant | MendeliKABS Europe Ltd |
| Therapeutic indication | Treatment of hereditary tyrosinemia type 1 |
| More information | CHMP summary of positive opinion for Nitisinone MendeliKABS |
| Name of medicine | Imraldi |
|---|---|
| INN | adalimumab |
| Marketing-authorisation applicant | Samsung Bioepis UK Limited (SBUK) |
| Therapeutic indication | Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, paediatric plaque psoriasis, hidradenitis suppurativa, Crohn's disease, paediatric Crohn's disease, ulcerative colitis and uveitis |
| More information | CHMP summary of positive opinion for Imraldi |
| Name of medicine | Adlumiz |
|---|---|
| INN | anamorelin hydrochloride |
| Marketing-authorisation applicant | Helsinn Birex Pharmaceuticals Ltd |
| Therapeutic indication | Treatment of anorexia, cachexia or unintended weight loss in patients with non-small cell lung cancer |
| More information | Questions and answers on refusal of the marketing authorisation for Adlumiz (anamorelin hydrochloride) |
| Name of medicine | Human IGG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech |
|---|---|
| INN | human IgG1 monoclonal antibody specific for human interleukin-1 alpha |
| Marketing-authorisation applicant | XBiotech Germany GmbH |
| Therapeutic indication | Treatment of metastatic colorectal cancer |
| More information | Questions and answers on refusal of the marketing authorisation for Human IgG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech |
| Name of medicine | Masipro |
|---|---|
| INN | masitinib |
| Marketing-authorisation applicant | AB Science |
| Therapeutic indication | Treatment of mastocytosis |
| More information | Questions and answers on refusal of the marketing authorisation for Masipro (masitinib) |
| Name of medicine | Faslodex |
|---|---|
| INN | fulvestrant |
| Marketing-authorisation holder | AstraZeneca UK Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Faslodex |
| Name of medicine | Harvoni |
|---|---|
| INN | ledipasvir / sofosbuvir |
| Marketing-authorisation holder | Gilead Sciences International Ltd |
| More information | Harvoni |
| Name of medicine | Kaletra |
|---|---|
| INN | lopinavir / ritonavir |
| Marketing-authorisation holder | AbbVie Ltd. |
| More information | CHMP post-authorisation summary of positive opinion for Kaletra |
| Name of medicine | Mimpara |
|---|---|
| INN | cinacalcet |
| Marketing-authorisation holder | Amgen Europe B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Mimpara (X-55-G) |
| Name of medicine | Orencia |
|---|---|
| INN | abatacept |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Orencia |
| Name of medicine | Soliris |
|---|---|
| INN | eculizumab |
| Marketing-authorisation holder | Alexion Europe SAS |
| More information | CHMP post-authorisation summary of positive opinion for Soliris |
| Name of medicine | SonoVue |
|---|---|
| INN | sulphur hexafluoride |
| Marketing-authorisation holder | Bracco International B.V. |
| More information | CHMP post-authorisation summary of positive opinion for SonoVue |
| Name of medicine | Stivarga |
|---|---|
| INN | regorafenib |
| Marketing-authorisation holder | Bayer Pharma AG |
| More information | CHMP post-authorisation summary of positive opinion for Stivarga (II-20) |
| Name of medicine | Victoza |
|---|---|
| INN | liraglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Victoza (II-42) |
| Name of medicine | Symbioflor 2 and associated names |
|---|---|
| INN | Escherichia coli bacteria (cells and autolysate) |
| Marketing-authorisation holder | Symbiopharm GmbH |
| More information | EMA recommends that Symbioflor 2 can continue to be used for irritable bowel syndrome |
| Name of medicine | Alcover 750 mg, 1250 mg, 1750 mg granules |
|---|---|
| INN | sodium oxybate |
| Marketing-authorisation holder | D&A Pharma |
| More information | Questions and answers on Alcover 750 mg, 1250 mg, 1750 mg granules |
| Name of medicine | Elmisol |
|---|---|
| INN | levamisole |
| Marketing-authorisation holder | ACE Pharmaceuticals BV |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Elmisol (levamisole) |
| Name of medicine | Zafiride |
|---|---|
| INN | NGR-human tumour necrosis factor alpha |
| Marketing-authorisation holder | MolMed SpA |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Zafiride (NGR-human tumour necrosis factor alpha) |