Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 19-22 October 2015
NewsHuman
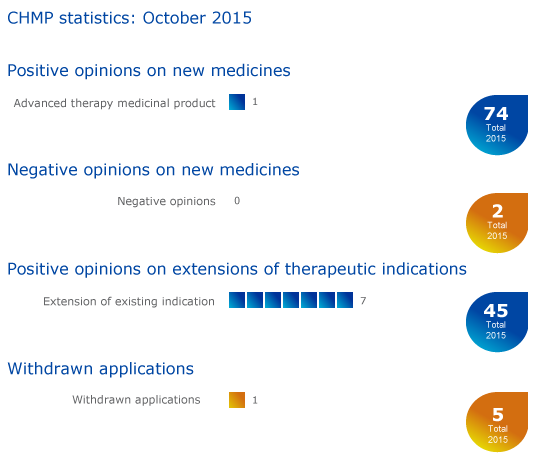
Advanced therapy medicinal product for melanoma receives positive opinion
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended an advanced therapy medicine for marketing authorisation at its October 2015 meeting.
Imlygic (talimogene laherparepvec) is a medicine for the treatment of adults with melanoma that cannot be removed by surgery and that has spread either to the surrounding area or to other areas of the body without affecting the bones, brain, lung or other internal organs. Imlygic is a first-in-class advanced therapy medicinal product (ATMP) derived from a virus that has been genetically engineered to infect and kill cancer cells. For more information on Imlygic, please see the press release in the grid below.
Seven recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Cubicin, Edurant, Emend, Volibris, Xalkori and two extensions of indication for Cosentyx.
Re-examination procedure concluded
The CHMP concluded a re-examination procedure, issuing a final negative opinion for Heparesc (human heterologous liver cells). A question-and-answer document on this opinion is available below.
Inductos to be suspended in the EU
The Committee recommended the suspension of Inductos, an implant used to help new bone develop in patients with spinal disc problems and leg fractures, due to manufacturing issues. Although no risk to patients has been identified, Inductos will remain suspended until issues with the manufacturing site for one of its components (an absorbable sponge) are resolved. For more information, please see the public health communication in the grid below.
Outcome of periodic safety review: Cellcept
The CHMP warned that the transplant medicine mycophenolate (authorised centrally as CellCept and nationally under various names) must not be used in pregnancy unless no alternative is available. This follows a routine re-assessment of the benefits and risks of these medicines, which provided updated evidence on the risk of birth defects and spontaneous abortions when pregnant women were exposed to these medicines. For more information, please see the public health communication in the grid below.
New advice for doctors and patients on Tecfidera
The CHMP gave new advice for doctors and patients to minimise the risk of progressive multifocal leukoencephalopathy (PML) in patients treated with the multiple sclerosis medicine Tecfidera (dimethyl fumarate). For more information, please see the public health communication in the grid below.
Update to safety information for medicines for the treatment of HIV infection
The CHMP has updated the advice on the risk of body fat changes and lactic acidosis with medicines for the treatment of human immunodeficiency virus (HIV) infection. As a result, HIV medicines will no longer require a warning concerning fat redistribution in their product information, and a number of medicines of the class 'nucleoside and nucleotide analogues' will no longer require a warning about lactic acidosis. For more information, please see the public health communication in the grid below.
Withdrawal of application
An application for marketing authorisation for VeraSeal (human fibrinogen / human thrombin) has been withdrawn. A question-and-answer document on this withdrawal is available below.
Agenda and minutes
The agenda of the October 2015 meeting is published on EMA's website. Minutes of the September 2015 CHMP meeting will be published next week.
CHMP statistics
Key figures from the October 2015 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's October 2015 meeting, is available in the grid below.
CHMP highlights - October 2015
| Name of medicine | Imlygic |
|---|---|
| International non-proprietary name (INN) | talimogene laherparepvec |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Therapeutic indication | Treatment of adults with melanoma |
| More information |
CHMP summary of opinion for Imlygic
Press release: First oncolytic immunotherapy medicine recommended for approval |
| Name of medicine | Heparesc |
|---|---|
| INN | human heterologous liver cells |
| Marketing-authorisation applicant | Cytonet GmbH Co KG |
| Therapeutic indication | Treatment of urea cycle disorders |
| More information | Questions and answers on refusal of the marketing authorisation for Heparesc (human heterologous liver cells) |
| Name of medicine | Cosentyx |
|---|---|
| INN | secukinumab |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Cosentyx |
| Name of medicine | Cubicin |
|---|---|
| INN | daptomycin |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Cubicin |
| Name of medicine | Edurant |
|---|---|
| INN | rilpivirine |
| Marketing-authorisation holder | Janssen-Cilag International N.V. |
| More information | CHMP post-authorisation summary of positive opinion for Edurant |
| Name of medicine | Emend |
|---|---|
| INN | aprepitant |
| Marketing-authorisation holder | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Emend |
| Name of medicine | Volibris |
|---|---|
| INN | ambrisentan |
| Marketing-authorisation holder | Glaxo Group Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Volibris |
| Name of medicine | Xalkori |
|---|---|
| INN | crizotinib |
| Marketing-authorisation holder | Pfizer Limited |
| More information | CHMP post-authorisation summary of positive opinion for Xalkori |
| Name of medicine | Antiretroviral medicinal products |
|---|---|
| More information | Updated advice on body fat changes and lactic acidosis with HIV medicines |
| Name of medicine | Cellcept |
|---|---|
| More information | EMA recommends additional measures to prevent use of mycophenolate in pregnancy |
| Name of medicine | Inductos |
|---|---|
| More information | Inductos to be suspended in the EU |
| Name of medicine | Tecfidera |
|---|---|
| More information | Updated recommendations to minimise the risk of the rare brain infection PML with Tecfidera |
| Name of medicine | VeraSeal |
|---|---|
| INN | human fibrinogen, human thrombin |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for VeraSeal (human fibrinogen / human thrombin) |