Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 20-23 June 2016
NewsHuman
Six new medicines, including one cell-based therapy, recommended for approval
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended six new medicines, including one advanced therapy medicinal product (ATMP), for approval at its June meeting.
The CHMP recommended granting a conditional marketing authorisation for the ATMP Zalmoxis as an adjunctive, or add-on, treatment for adult patients receiving a haploidentical haematopoietic stem cell transplant (HSCT) for types of blood cancer to aid immune reconstitution and reduce the risk of graft-versus-host disease. Zalmoxis has an orphan designation. For more information, please see the press release in the grid below.
The Committee also recommended granting a marketing authorisation for Cinqaero (reslizumab) as an add-on treatment for adult patients with severe eosinophilic asthma.
The generic medicine Atazanavir Mylan (atazanavir) was recommended by the CHMP for the treatment of human immunodeficiency virus-1 (HIV-1) infections.
Three hybrid applications received positive recommendations from the Committee. Aerivio Spiromax and Airexar Spiromax, both containing salmeterol xinafoate and fluticasone propionate, were recommended for the treatment of asthma and chronic obstructive pulmonary disorder (COPD). Nordimet (methotrexate) was recommended for the treatment of active rheumatoid arthritis, juvenile idiopathic arthritis and severe psoriatic arthritis. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data.
Request for re-examination of CHMP recommendation
The applicant for Ninlaro has requested a re-examination of the CHMP's negative opinion for this medicine adopted at the May 2016 meeting. Upon receipt of the grounds for re-examination, the CHMP will re-examine this opinion and issue a final opinion.
Seven recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Cervarix, Ilaris, Keytruda, Nevanac, RoActemra, Ryzodeg and Zontivity.
Negative opinion on extension of indication
The CHMP adopted a negative opinion on the request for an extension of therapeutic indication for Arzerra. A questions and answers document is available in the grid below.
Start of review: Pharmaceutics International Inc
The CHMP started a review of medicines manufactured by Pharmaceutics International Inc, USA. This follows an inspection in February 2016 which highlighted several shortcomings in relation to good manufacturing practice (GMP). For more information, please see the start of referral document in the grid below.
Outcome of Alkem Laboratories Ltd review
The CHMP recommended the suspension of a medicine (Riluzole Alkem), for which studies were conducted at the Alkem Laboratories Ltd site in Taloja, India, and has required companies to provide new data for another medicine before it can be authorised in the EU. The recommendations follow a joint routine inspection by German and Dutch authorities in March 2015. For more information, please see the public health communication in the grid below.
Review of a safety signal for Adempas
The Committee recommended that Adempas (riociguat) should not be used in patients with symptomatic pulmonary hypertension associated with idiopathic interstitial pneumonia or PH-IIP (high blood pressure in lung arteries caused by a lung disease called idiopathic interstitial pneumonia). These recommendations have been issued in the context of a review of a safety signal. For more information, please see the public health communication in the grid below.
Update to product information for Noxafil
The CHMP has warned that Noxafil (posaconazole) tablets and oral suspension have different doses and are not interchangeable. The product information for the medicine is to be updated to strengthen warnings that the two dose forms given by mouth cannot be simply interchanged at the same dose. For more information, please see the public health communication in the grid below.
Withdrawals of applications
Applications for marketing authorisations for Alendronic Acid/Colecalciferol Mylan (alendronic acid/colecalciferol), Arikayce (amikacin), Docetaxel Sun (docetaxel) and Kyndrisa (drisapersen) have been withdrawn. Questions-and-answers documents on these withdrawals are available in the grid below.
Agenda and minutes
The agenda of the June 2016 meeting is published on EMA's website. Minutes of the May 2016 CHMP meeting will be published next week.
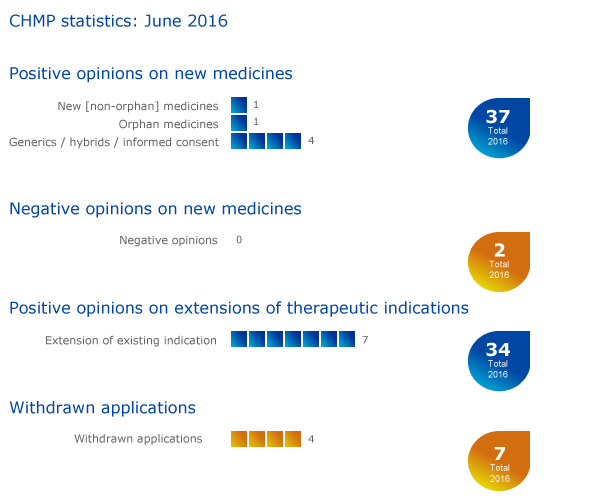
CHMP statistics
Key figures from the June 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's June 2016 meeting, is available in the grid below.
| Name of medicine | Cinqaero |
|---|---|
| International non-proprietary name (INN) | reslizumab |
| Marketing-authorisation applicant | Teva Pharmaceuticals Limited |
| Therapeutic indication | Treatment of severe eosinophilic asthma |
| More information | CHMP summary of positive opinion for Cinqaero |
| Name of medicine | Zalmoxis |
|---|---|
| INN | allogeneic T cells genetically modified with a retroviral vector encoding for a truncated form of the human low affinity nerve growth factor receptor (?LNGFR) and the herpes simplex I virus thymidine kinase (HSV-TK Mut2) |
| Marketing-authorisation applicant | MolMed SpA |
| Therapeutic indication | Treatment in haploidentical haematopoietic stem cell transplantation |
| More information |
Press release: New cell-based therapy to support stem cell transplantation in patients with high-risk blood cancer |
| Name of medicine | Atazanavir Mylan |
|---|---|
| INN | atazanavir |
| Marketing-authorisation applicant | Mylan S.A.S |
| Therapeutic indication | Treatment of HIV-1 infection |
| More information |
| Name of medicine | Aerivio Spiromax |
|---|---|
| INN | fluticasone propionate / salmeterol |
| Marketing-authorisation applicant | Teva B.V. |
| Therapeutic indication | Treatment of asthma and COPD |
| More information |
| Name of medicine | Airexar Spiromax |
|---|---|
| INN | fluticasone propionate / salmeterol |
| Marketing-authorisation applicant | Teva B.V. |
| Therapeutic indication | Treatment of asthma and COPD |
| More information |
| Name of medicine | Nordimet |
|---|---|
| INN | methotrexate |
| Marketing-authorisation applicant | Nordic Group B.V. |
| Therapeutic indication | Treatment of active rheumatoid arthritis, juvenile idiopathic arthritis and severe psoriatic arthritis |
| More information | CHMP summary of opinion for Nordimet |
| Name of medicine | Cervarix |
|---|---|
| INN | human papillomavirus vaccine [types 16, 18] (recombinant, adjuvanted, adsorbed) |
| Marketing-authorisation holder | GlaxoSmithKline Biologicals |
| More information | CHMP post-authorisation summary of positive opinion for Cervarix |
| Name of medicine | Ilaris |
|---|---|
| INN | canakinumab |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Ilaris |
| Name of medicine | Keytruda |
|---|---|
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Keytruda |
| Name of medicine | Nevanac |
|---|---|
| INN | nepafenac |
| Marketing-authorisation holder | Alcon Laboratories (UK) Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Nevanac |
| Name of medicine | RoActemra |
|---|---|
| INN | tocilizumab |
| Marketing-authorisation holder | Roche Registration Limited |
| More information | CHMP post-authorisation summary of positive opinion for RoActemra (II/57) |
| Name of medicine | Ryzodeg |
|---|---|
| INN | insulin degludec / insulin aspart |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Ryzodeg |
| Name of medicine | Zontivity |
|---|---|
| INN | vorapaxar |
| Marketing-authorisation holder | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Zontivity (II/0005) |
| Name of medicine | Arzerra |
|---|---|
| INN | ofatumumab |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information |
| Name of medicine | Pharmaceutics International Inc. |
|---|---|
| More information | Start of review of medicines manufactured at Pharmaceutics International Inc., USA |
| Name of medicine | Alkem Laboratories Limited |
|---|---|
| More information | Studies from Alkem Laboratories Ltd cannot be used to support medicines approval in the EU |
| Name of medicine | Adempas |
|---|---|
| INN | riociguat |
| Marketing-authorisation holder | Bayer Pharma AG |
| More information | Adempas not for use in patients with pulmonary hypertension caused by idiopathic interstitial pneumonia |
| Name of medicine | Noxafil |
|---|---|
| INN | posaconazole |
| Marketing-authorisation holder | Merck Sharp & Dohme Ltd |
| More information | EMA warns that Noxafil tablets and oral suspension have different doses and are not interchangeable |
| Name of medicine | Alendronic Acid/Colecalciferol Mylan |
|---|---|
| INN | alendronic acid / colecalciferol |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Alendronic Acid/Colecalciferol Mylan (alendronic acid and colecalciferol) |
| Name of medicine | Arikayce |
|---|---|
| INN | amikacin |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Arikayce (amikacin) |
| Name of medicine | Docetaxel SUN |
|---|---|
| INN | docetaxel |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Docetaxel Sun (docetaxel) |
| Name of medicine | Kyndrisa |
|---|---|
| INN | drisapersen |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Kyndrisa (drisapersen) |