Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 21-24 September 2015
NewsHuman
19 new medicines, of which two have been fast-tracked, receive positive opinions from the Committee
At its busiest meeting of 2015 so far, the Committee for Medicinal Products for Human Use (CHMP) recommended 19 new medicines for marketing authorisation.
The Committee recommended granting a marketing authorisation, under accelerated assessment, for Praxbind (idarucizumab) as a specific antidote to the anticoagulant medicine Pradaxa (dabigatran etexilate), when rapid reversal of its effect is required. Praxbind is to be used when a patient taking Pradaxa needs to undergo an emergency surgery or when life-threatening or uncontrolled bleeding occurs. For more information on Praxbind, please see the press release in the grid below.
Entresto (sacubitril / valsartan) was recommended for the treatment of symptomatic chronic heart failure with reduced ejection fraction - a condition where the heart muscle does not contract effectively and less oxygen-rich blood is pumped out to the body. For more information on Entresto, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Kyprolis (carfilzomib) to treat patients with multiple myeloma whose disease has relapsed after receiving at least one prior course of therapy. Kyprolis is for use in combination with the medicines lenalidomide and dexamethasone. Kyprolis has an orphan designation and was reviewed under accelerated assessment. For more information on Kyprolis, please see the press release in the grid below.
The CHMP recommended granting a conditional marketing authorisation for Blincyto (blinatumomab) for the treatment of Philadelphia chromosome-negative acute lymphoblastic leukaemia. Conditional marketing authorisations are one of the mechanisms put in place by the Agency to facilitate market access for medicines that fulfill unmet medical needs. Blincyto has an orphan designation.
Cotellic (cobimetinib) was recommended by the Committee for the treatment of metastatic melanoma.
Genvoya (elvitegravir / cobicistat / emtricitabine / tenofovir alafenamide) received a positive opinion from the Committee for the treatment of Human Immunodeficiency Virus (HIV) infection.
The CHMP recommended Nucala (mepolizumab) for the treatment of asthma and Orkambi (lumacaftor / ivacaftor) for the treatment of cystic fibrosis. Orkambi has an orphan designation.
Numient (levodopa / carbidopa) received a positive opinion from the Committee for the treatment of Parkinson's disease.
The Committee also granted positive opinions for Ionsys (fentanyl) for the treatment of post-operative pain, as well as Elocta (efmoroctocog alfa) for the treatment of haemophilia A. Elocta has an orphan designation.
Ravicti (glycerol phenylbutyrate), which also has an orphan designation, received a positive opinion from the Committee for the treatment of urea cycle disorders.
Ebymect (dapagliflozin / metformin) and Edistride (dapagliflozin) received positive opinions for the treatment of type 2 diabetes mellitus. These medicines were submitted as informed consent applications. This means that the applications make use of data from the dossier of a previously authorised medicine, with the marketing authorisation holder of that medicine giving consent for the use of their data in the application.
Five generic medicines received positive opinions from the CHMP: Aripiprazole Accord (aripiprazole) for the treatment of schizophrenia and the prevention and treatment of manic episodes of bipolar 1 disorder, Ciambra (pemetrexed), Pemetrexed Hospira (pemetrexed) and Pemetrexed Medac (pemetrexed) all to be used for the treatment of unresectable malignant pleural mesothelioma and locally advanced or metastatic non-small cell lung cancer, and Cinacalcet Mylan (cinacalcet) for the treatment of hyperparathyroidism and parathyroid carcinoma.
Six recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Eylea, Gilenya, Kalydeco, Opdivo, Rebetol and Vidaza.
Agenda and minutes
The agenda of the September 2015 meeting is published on EMA's website. Minutes of the July 2015 CHMP meeting will be published next week.
CHMP statistics
Key figures from the September 2015 CHMP meeting are represented in the graphic below.1
More information on this, and all other outcomes of the CHMP's September 2015 meeting, is available in the grid below.
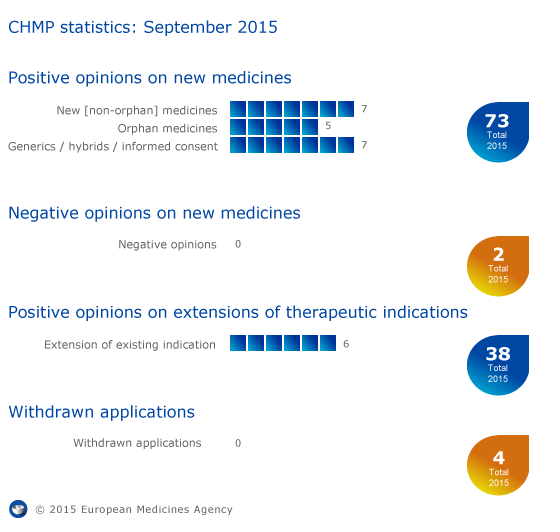
CHMP statistics: September 2015
1In addition to the opinions on new medicines, the CHMP also adopted a revised positive opinion for Kolbam (cholic acid) for the treatment of inborn errors in primary bile acid synthesis. The revised opinion follows the annulment on 11 June 2015 of the marketing authorisation for Kolbam in the European Union following a judgment of the General Court.
| Name of medicine | Blincyto |
|---|---|
| International non-proprietary name (INN) | blinatumomab |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Therapeutic indication | Treatment of adults with Philadelphia chromosome negative relapsed or refractory B-precursor acute lymphoblastic leukaemia |
| More information | CHMP summary of opinion for Blincyto |
| Name of medicine | Cotellic |
|---|---|
| INN | cobimetinib |
| Marketing-authorisation applicant | Roche Registration Ltd |
| Therapeutic indication | Treatment of metastatic melanoma |
| More information | CHMP summary of positive opinion for Cotellic |
| Name of medicine | Elocta |
|---|---|
| INN | efmoroctocog alfa |
| Marketing-authorisation applicant | Biogen Idec Ltd |
| Therapeutic indication | Treatment of haemophilia A |
| More information | CHMP summary of positive opinion for Elocta |
| Name of medicine | Entresto |
|---|---|
| INN | sacubitril / valsartan |
| Marketing-authorisation applicant | Novartis Europharm Ltd |
| Therapeutic indication | Treatment of heart failure (NYHA class II-IV) |
| More information |
CHMP summary of positive opinion Entresto
Press release: New medicine to treat heart failure recommended for approval |
| Name of medicine | Genvoya |
|---|---|
| INN | elvitegravir, cobicitat, emtricitabine, tenofovir alafenamide |
| Marketing-authorisation applicant | Gilead Sciences International Ltd |
| Therapeutic indication | Treatment of Human Immunodeficiency Virus (HIV) infection |
| More information | CHMP summary of positive opinion for Genvoya |
| Name of medicine | Ionsys |
|---|---|
| INN | fentanyl |
| Marketing-authorisation applicant | Incline Therapeutics Europe Ltd |
| Therapeutic indication | Treatment of post-operative pain |
| More information |
| Name of medicine | Kyprolis |
|---|---|
| INN | carfilzomib |
| Marketing-authorisation applicant | Amgen Europe B.V. |
| Therapeutic indication | Treatment of multiple myeloma |
| More information |
CHMP summary of positive opinion Kyprolis
Press release: New treatment option for patients with rare blood cancer |
| Name of medicine | Nucala |
|---|---|
| INN | mepolizumab |
| Marketing-authorisation applicant | GlaxoSmithKline Trading Services |
| Therapeutic indication | Treatment of asthma |
| More information | CHMP summary of positive opinion Nucala |
| Name of medicine | Numient |
|---|---|
| INN | levodopa / carbidopa |
| Marketing-authorisation applicant | Impax Laboratories Netherlands BV |
| Therapeutic indication | Treatment of Parkinson's disease |
| More information | CHMP summary of positive opinion Numient |
| Name of medicine | Orkambi |
|---|---|
| INN | lumacaftor / ivacaftor |
| Marketing-authorisation applicant | Vertex Pharmaceuticals (U.K.) Ltd |
| Therapeutic indication | Treatment of cystic fibrosis |
| More information | CHMP summary of positive opinion Orkambi |
| Name of medicine | Praxbind |
|---|---|
| INN | idarucizumab |
| Marketing-authorisation applicant | Boehringer Ingelheim International GmbH |
| Therapeutic indication | Prevention and treatment of dabigatran associated haemorrhage |
| More information |
CHMP summary of positive opinion for Praxbind
Press release: EMA fast-tracks antidote to anticoagulant Pradaxa |
| Name of medicine | Ravicti |
|---|---|
| INN | glycerol phenylbutyrate |
| Marketing-authorisation applicant | Horizon Therapeutics Limited |
| Therapeutic indication | Treatment of patients with urea cycle disorders |
| More information | CHMP summary of positive opinion for Ravicti |
| Name of medicine | Ebymect |
|---|---|
| INN | dapagliflozin / metformin |
| Marketing-authorisation applicant | AstraZeneca AB |
| Therapeutic indication | Diabetes mellitus |
| More information | CHMP summary of positive opinion Ebymect |
| Name of medicine | Edistride |
|---|---|
| INN | dapagliflozin |
| Marketing-authorisation applicant | AstraZeneca AB |
| Therapeutic indication | Diabetes mellitus |
| More information | CHMP summary of positive opinion Edistride |
| Name of medicine | Aripiprazole Accord |
|---|---|
| INN | aripiprazole |
| Marketing-authorisation applicant | Accord Healthcare Ltd |
| Therapeutic indication | Treatment of schizophrenia and treatment and prevention of manic episodes in bipolar I disorder |
| More information | CHMP summary of positive opinion Aripiprazole Accord |
| Name of medicine | Ciambra |
|---|---|
| INN | pemetrexed |
| Marketing-authorisation applicant | Menarini International Operations Luxembourg S.A. |
| Therapeutic indication | Treatment of malignant pleural mesothelioma and non-small cell lung cancer |
| More information | CHMP summary of positive opinion for Ciambra |
| Name of medicine | Cinacalcet Mylan |
|---|---|
| INN | cinacalcet |
| Marketing-authorisation applicant | Mylan S.A.S |
| Therapeutic indication | Treatment of hyperparathyroidism and parathyroid carcinoma |
| More information | CHMP summary of positive opinion for Cinacalcet Mylan |
| Name of medicine | Pemetrexed Hospira |
|---|---|
| INN | pemetrexed |
| Marketing-authorisation applicant | Hospira UK Limited |
| Therapeutic indication | Treatment of malignant pleural mesothelioma and non-small cell lung cancer (excluding predominantly squamous cell histology) |
| More information | CHMP summary of positive opinion Pemetrexed Hospira |
| Name of medicine | Pemetrexed medac |
|---|---|
| INN | pemetrexed |
| Marketing-authorisation applicant | medac Gesellschaft fur klinische Spezialpraparate mbH |
| Therapeutic indication | Treatment of malignant pleural mesothelioma and non-small cell lung cancer |
| More information | CHMP summary of opinion for Pemetrexed medac |
| Name of medicine | Eylea |
|---|---|
| INN | aflibercept |
| Marketing-authorisation holder | Bayer Pharma AG |
| More information | CHMP post-authorisation summary of positive opinion for Eylea |
| Name of medicine | Gilenya |
|---|---|
| INN | fingolimod |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Gilenya |
| Name of medicine | Kalydeco |
|---|---|
| INN | ivacaftor |
| Marketing-authorisation holder | Vertex Pharmaceuticals (U.K.) Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Kalydeco |
| Name of medicine | Opdivo |
|---|---|
| INN | nivolumab |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Opdivo |
| Name of medicine | Rebetol |
|---|---|
| INN | ribavirin |
| Marketing-authorisation holder | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Rebetol |
| Name of medicine | Vidaza |
|---|---|
| INN | azacitidine |
| Marketing-authorisation holder | Celgene Europe Limited |
| More information | CHMP post-authorisation summary of positive opinion for Vidaza-II-30 |
| Name of medicine | Kolbam |
|---|---|
| INN | cholic acid |
| Marketing-authorisation applicant | Retrophin Europe Ltd |
| Therapeutic indication | Treatment of inborn errors of primary bile acid synthesis |
| More information | CHMP summary of positive opinion Kolbam |