Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 25-28 April 2016
NewsHuman
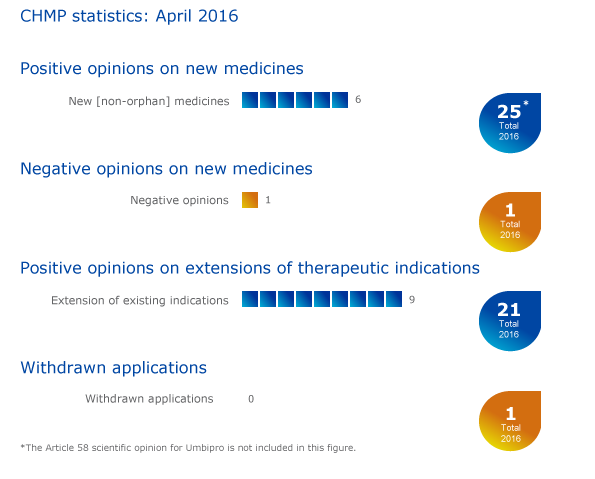
Six medicines, including one new antibacterial recommended for authorisation in the EU; antiseptic gel for newborns receives positive scientific opinion for use outside the EU
At its April meeting, the Committee for Medicinal Products for Human Use (CHMP) gave a positive scientific opinion for Umbipro (chlorhexidine digluconate), an antiseptic gel to prevent umbilical cord infections (omphalitis) in newborn babies, for use in countries outside the European Union (EU).
Umbipro was submitted to the European Medicines Agency (EMA) under a regulatory procedure (Article 58) that allows the Agency to assess the quality, safety and efficacy of a medicine and give an opinion on its benefit-risk balance when used in low-income countries outside the EU. For more information on Umbipro and the Article 58 procedure, please see the press release in the grid below.
In addition, six medicines were recommended for marketing authorisation in the EU. These recommendations will be sent to the European Commission for the adoption of a decision on an EU-wide marketing authorisation:
Zavicefta (ceftazidime / avibactam), a new treatment option against multi-drug resistant bacteria was recommended for approval by the Committee. The medicine is to be used in adult patients with intra-abdominal infection, urinary tract infection, as well as pneumonia acquired in a hospital setting. It is also indicated for the treatment of adults with infections caused by certain Gram-negative bacteria, for which there are only limited treatment options. For more information on Zavicefta, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Zinbryta (daclizumab) for the treatment of relapsing forms of multiple sclerosis.
Ongentys (opicapone) received a positive opinion from the Committee for the treatment of Parkinson's disease and motor fluctuations.
The CHMP gave a positive opinion for Odefsey (emtricitabine / rilpivirine / tenofovir alafenamide) for the treatment of human immunodeficiency virus type 1 (HIV-1) infection.
Enzepi (pancreas powder) received a positive opinion for the treatment of exocrine pancreatic insufficiency.
EndolucinBeta (lutetium (177 lu) chloride) also received a positive opinion. It is a radiopharmaceutical precursor that has to be combined with another medicine, a carrier medicine, in a process called radiolabelling before administration. The carrier medicine then takes EndolucinBeta to the disease site in the body where it gives off beta-radiation, allowing a localised radiation effect.
Negative opinion on new medicine
The CHMP adopted a negative opinion for Sialanar (glycopyrronium bromide). Sialanar was intended for the treatment of persistent drooling in children and adolescents with neurological conditions. For more information, please see the questions-and-answers document in the grid below.
Nine recommendations on extensions of therapeutic indications
The Committee recommended adding treatment of follicular lymphoma to the approved indication for Gazyvaro. For more information on Gazyvaro, please see the press release in the grid below.
The CHMP also recommended extensions of indications for Afinitor, Avastin, Ferriprox, HyQvia, Imbruvica, Reyataz, Victoza and Zinforo.
Start of review on the conduct of studies at Semler Research Centre Private Ltd
The CHMP has started a review of medicines for which studies have been conducted at Semler Research Centre Private Ltd in Bangalore, India. This follows inspections by the FDA and the World Health Organization which raised serious concerns over data generated at Semler's sites. For more information, please see the start of referral documents in the grid below.
Outcome of review of inhaled corticosteroids for chronic obstructive pulmonary disease
The Committee completed its review of the known risk of pneumonia (lung infection) in patients who take inhaled corticosteroid medicines to treat chronic obstructive pulmonary disease (COPD). The review confirmed the known risk of pneumonia with these products, but did not find conclusive evidence of differences in this risk between different products in this class. For more information, please see the public health communication in the grid below.
Agenda and minutes
The agenda of the April 2016 meeting is published on EMA's website. Minutes of the March 2016 CHMP meeting will be published next week.
CHMP statistics
Key figures from the April 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's April 2016 meeting, is available in the grid below.
| Name of medicine | EndolucinBeta |
|---|---|
| INN | lutetium (177 lu) chloride |
| Marketing-authorisation applicant | ITG Isotope Technologies Garching GmbH |
| Therapeutic indication | Radiolabelling of carrier molecules that have been specifically developed and authorised for radiolabelling with Lutetium (177Lu) chloride |
| More information | CHMP summary of opinion for EndolucinBeta |
| Name of medicine | Enzepi |
|---|---|
| INN | pancreas powder |
| Marketing-authorisation applicant | Aptalis Pharma SAS |
| Therapeutic indication | Treatment of exocrine pancreatic insufficiency |
| More information | CHMP summary of opinion for Enzepi |
| Name of medicine | Odefsey |
|---|---|
| INN | emtricitabine / rilpivirine / tenofovir alafenamide |
| Marketing-authorisation applicant | Gilead Sciences International Ltd |
| Therapeutic indication | Treatment of HIV-1 |
| More information | CHMP summary of opinion for Odefsey |
| Name of medicine | Ongentys |
|---|---|
| INN | opicapone |
| Marketing-authorisation applicant | Bial - Portela & Cª, S.A. |
| Therapeutic indication | Parkinson's disease and motor fluctuations |
| More information | CHMP summary of positive opinion for Ongentys |
| Name of medicine | Umbipro |
|---|---|
| INN | chlorhexidine |
| Marketing-authorisation applicant | GlaxoSmithKline Trading Services |
| Therapeutic indication | Prophylaxis of omphalitis |
| More information |
Press release: Boosting care for newborn babies in low-income countries |
| Name of medicine | Zavicefta |
|---|---|
| INN | ceftazidime / avibactam |
| Marketing-authorisation applicant | AstraZeneca AB |
| Therapeutic indication | Treatment in adults of complicated Intra-Abdominal Infection, complicated urinary tract infection, including pyelonephritis, hospital-acquired pneumonia, including ventilator associated pneumonia, infections due to aerobic Gram-negative organisms in patients with limited treatment options |
| More information |
CHMP summary of opinion for Zavicefta
Press release: New medicine to help in the fight against antimicrobial resistance |
| Name of medicine | Zinbryta |
|---|---|
| INN | daclizumab |
| Marketing-authorisation applicant | Biogen Idec Ltd |
| Therapeutic indication | Treatment of relapsing forms of multiple sclerosis |
| More information | CHMP summary of opinion for Zinbryta |
| Name of medicine | Sialanar |
|---|---|
| INN | glycopyrronium bromide |
| Marketing-authorisation applicant | Proveca Limited |
| Therapeutic indication | Treatment of sialorrhoea |
| More information | Questions and answers on refusal of the marketing authorisation for Sialanar (glycopyrronium bromide) |
| Name of medicine | Afinitor |
|---|---|
| INN | everolimus |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Afinitor |
| Name of medicine | Avastin |
|---|---|
| INN | bevacizumab |
| Marketing-authorisation holder | Roche Registration Limited |
| More information | CHMP post-authorisation summary of positive opinion for Avastin |
| Name of medicine | Ferriprox |
|---|---|
| INN | deferiprone |
| Marketing-authorisation holder | Apotex Europe BV |
| More information | CHMP post-authorisation summary of positive opinion for Ferriprox |
| Name of medicine | Gazyvaro |
|---|---|
| INN | obinutuzumab |
| Marketing-authorisation holder | Roche Registration Limited |
| More information |
CHMP post-authorisation summary of positive opinion for Gazyvaro
Press release: New treatment for rare white blood cell cancer |
| Name of medicine | HyQvia |
|---|---|
| INN | human normal immunoglobulin |
| Marketing-authorisation holder | Baxalta Innovations GmbH |
| More information | CHMP post-authorisation summary of positive opinion for HyQvia |
| Name of medicine | Imbruvica |
|---|---|
| INN | ibrutinib |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information | CHMP post-authorisation summary of positive opinion for Imbruvica |
| Name of medicine | Reyataz |
|---|---|
| INN | atazanavir / atazanavir sulfate |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Reyataz |
| Name of medicine | Victoza |
|---|---|
| INN | liraglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | CHMP post-authorisation summary of positive opinion for Victoza |
| Name of medicine | Zinforo |
|---|---|
| INN | ceftaroline fosamil |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | CHMP post-authorisation summary of positive opinion for Zinforo |
| Name of medicine | Semler |
|---|---|
| More information | Start of a review on the conduct of studies at Semler Research Centre Private Ltd, Bangalore, India |
| Name of medicine | Inhaled corticosteroids (ICS)-containing medicinal products indicated in the treatment of chronic obstructive pulmonary disease |
|---|---|
| More information | EMA completes review of inhaled corticosteroids for chronic obstructive pulmonary disease |
| Name of medicine | Novantrone and associated names |
|---|---|
| INN | mitoxantrone |
| Marketing-authorisation holder | MEDA group of companies and associated companies |
| More information | Questions and answers on Novantrone and associated names (mitoxantrone 2 mg/ml concentrate for solution for infusion) |