Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 27 - 30 March 2023
NewsHumanCOVID-19MedicinesVaccines
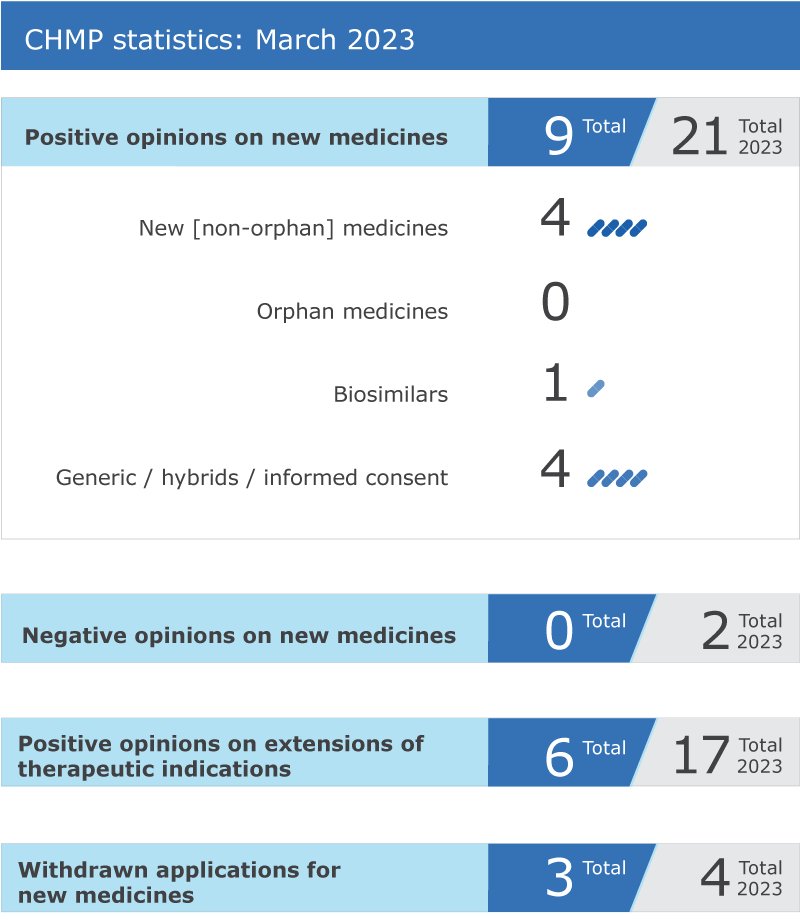
EMA’s human medicines committee (CHMP) recommended nine medicines for approval at its March 2023 meeting.
The CHMP recommended authorising the COVID-19 vaccine Bimervax (previously COVID-19 Vaccine HIPRA) as a booster in people aged 16 years and older who have previously been vaccinated with a mRNA COVID-19 vaccine. It is the eighth vaccine recommended in the European Union (EU) for protecting against COVID-19 and, together with the vaccines already authorised, will support vaccination campaigns in EU Member States during the pandemic. An overview of all the COVID-19 vaccines authorised in the EU is available on EMA’s website. See more information in the news announcement in the grid below.
The committee adopted a positive opinion for Briumvi (ublituximab) for the treatment of relapsing multiple sclerosis, a disease of the brain and spinal cord in which inflammation destroys the protective covering around nerves and the nerve themselves.
Omvoh (mirikizumab) received a positive opinion from the CHMP for the treatment of moderately to severely active ulcerative colitis, an inflammation of the large intestine causing ulceration and bleeding.
The committee recommended granting a paediatric-use marketing authorisation (PUMA) for Pedmarqsi (sodium thiosulfate) for the prevention of ototoxicity induced by cisplatin chemotherapy. Ototoxicity is the development of hearing or balance problems due to a medicine and Cisplatin is a chemotherapy used to treat several types of cancer. Pedmarqsi is indicated in patients from one month up to 18 years of age with localised, non-metastatic, solid tumours.
The committee adopted a positive opinion for Epysqli (eculizumab), a biosimilar medicine for the treatment of paroxysmal nocturnal haemoglobinuria, a rare disorder that leads to the premature destruction and impaired production of blood cells.
The CHMP gave a positive opinion to Qaialdo (spironolactone) for the management of refractory oedema, a persistent swelling which does not respond to the use of diuretics and sodium restriction. This medicine was submitted as a hybrid application, which relies in part on the results of pre-clinical tests and clinical trials of an already authorised reference product and in part on new data.
The committee adopted positive opinions for three generic medicines:
The committee recommended six extensions of indication for medicines that are already authorised in the EU: Breyanzi, Entresto and its duplicate Neparvis, Tenkasi, Ultomiris and Wegovy.
For Ultomiris, the committee also recommended a new route of administration with a new strength and pharmaceutical form.
Three applications for marketing authorisation were withdrawn:
Question-and-answer documents on the withdrawals of Feraheme and Raltegravir Viatris are available in the grid below. Onteeo is a duplicate of a medicine which is currently under evaluation.
The Agenda of the CHMP meeting 27-30 March 2023 of the March 2023 CHMP meeting is published on EMA's website. Minutes of the February 2023 CHMP meeting will be published in the coming weeks.
Key figures from the March 2023 CHMP meeting are represented in the graphic below.
| Name of medicine | Bimervax |
|---|---|
| Common name | COVID-19 vaccine |
| Marketing-authorisation applicant | Hipra Human Health SLU |
| Therapeutic indication | Immunisation to prevent COVID-19 caused by SARS-CoV-2 |
| More information | Bimervax: Pending EC decision
News: EMA recommends approval of Bimervax as a COVID-19 booster vaccine |
| Name of medicine | Briumvi |
|---|---|
| INN | ublituximab |
| Marketing-authorisation applicant | Propharma Group The Netherlands B.V. |
| Therapeutic indication | Treatment of relapsing forms of multiple sclerosis (RMS) (new active substance) |
| More information | Briumvi: Pending EC decision |
| Name of medicine | Omvoh |
|---|---|
| INN | mirikizumab |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Treatment of moderately to severely active ulcerative colitis (new active substance) |
| More information | Omvoh: Pending EC decision |
| Name of medicine | Pedmarqsi |
|---|---|
| INN | sodium thiosulfate |
| Marketing-authorisation applicant | Fennec Pharmaceuticals (EU) Limited |
| Therapeutic indication | For the prevention of ototoxicity induced by cisplatin (CIS) chemotherapy in patients 1 month to < 18 years of age with localized, non-metastatic, solid tumours (known active substance) |
| More information | Pedmarqsi: Pending EC decision |
| Name of medicine | Epysqli |
|---|---|
| INN | eculizumab |
| Marketing-authorisation applicant | Samsung Bioepis NL B.V. |
| Therapeutic indication | Treatment of paroxysmal nocturnal haemoglobinuria |
| More information | Epysqli: Pending EC decision |
| Name of medicine | Qaialdo |
|---|---|
| INN | spironolactone |
| Marketing-authorisation applicant | Nova Laboratories Ireland Limited |
| Therapeutic indication | Management of refractory oedema |
| More information | Qaialdo: Pending EC decision |
| Name of medicine | Dabigatran Etexilate Accord |
|---|---|
| INN | dabigatran etexilate |
| Marketing-authorisation applicant | Accord Healthcare S.L.U. |
| Therapeutic indication | Prevention of venous thromboembolic events |
| More information | Dabigatran Etexilate Accord: Pending EC decision |
| Name of medicine | Lacosamide Adroiq |
|---|---|
| INN | lacosamide |
| Marketing-authorisation applicant | Extrovis EU Ltd. |
| Therapeutic indication | Treatment of epilepsy |
| More information | Lacosamide Adroiq: Pending EC decision |
| Name of medicine | Sugammadex Adroiq |
|---|---|
| INN | sugammadex |
| Marketing-authorisation applicant | Extrovis EU Ltd. |
| Therapeutic indication | Reversal of neuromuscular blockade induced by rocuronium or vecuronium |
| More information | Sugammadex Adroiq: Pending EC decision |
| Name of medicine | Breyanzi |
|---|---|
| INN | lisocabtagene maraleucel / lisocabtagene maraleucel |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | Breyanzi: Pending EC decision |
| Name of medicine | Entresto |
|---|---|
| INN | sacubitril / valsartan |
| Marketing-authorisation holder | Novartis Europharm Limited |
| More information | Entresto: Pending EC decision |
| Name of medicine | Neparvis |
|---|---|
| INN | sacubitril / valsartan |
| Marketing-authorisation holder | Novartis Europharm Limited |
| More information | Neparvis : Pending EC decision |
| Name of medicine | Tenkasi |
|---|---|
| INN | oritavancin |
| Marketing-authorisation holder | Menarini International Operations Luxembourg S.A. |
| More information | Tenkasi: Pending EC decision |
| Name of medicine | Ultomiris |
|---|---|
| INN | ravulizumab |
| Marketing-authorisation holder | Alexion Europe SAS |
| More information | Ultomiris: Pending EC decision (II-32) |
| Name of medicine | Wegovy |
|---|---|
| INN | semaglutide |
| Marketing-authorisation holder | Novo Nordisk A/S |
| More information | Wegovy: Pending EC decision |
| Name of medicine | Ultomiris |
|---|---|
| INN | ravulizumab |
| Marketing-authorisation applicant | Alexion Europe SAS |
| More information | Ultomiris: Pending EC decision (X-27-G) |
| Name of medicine | Feraheme |
|---|---|
| INN | ferumoxytol |
| Marketing-authorisation applicant | Covis Pharma Europe B.V. |
| More information | Feraheme: Pending EC decision |
| Name of medicine | Onteeo |
|---|---|
| INN | tocilizumab |
| Marketing-authorisation applicant | Fresenius Kabi Deutschland GmbH |
| More information | Onteeo is a duplicate of a medicine which is currently under evaluation |
| Name of medicine | Raltegravir Viatris |
|---|---|
| INN | raltegravir potassium |
| Marketing-authorisation applicant | Viatris Limited |
| More information | Raltegravir Viatris: Pending EC decision |