Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 11-14 June 2019
NewsHumanMedicinesPharmacovigilance
EMA’s safety committee (PRAC) has started a review of leuprorelin medicines after reports indicated that handling errors during preparation and administration can cause some patients to receive insufficient amounts of their medicine, thus reducing the benefits of treatment.
The PRAC will now evaluate all available data and determine whether measures are needed to ensure that the medicines are prepared and administered appropriately.
While the review is ongoing, healthcare professionals should carefully follow the handling instructions for leuprorelin medicines. Patients prescribed these medicines who have any concerns should discuss them with their doctor.
More information is available below.
PRAC statistics: June 2019
The infographic below presents the total number of procedures discussed during the PRAC meeting in June 2019, as captured and detailed in the meeting Agenda - PRAC draft agenda of meeting 11-14 June 2019.
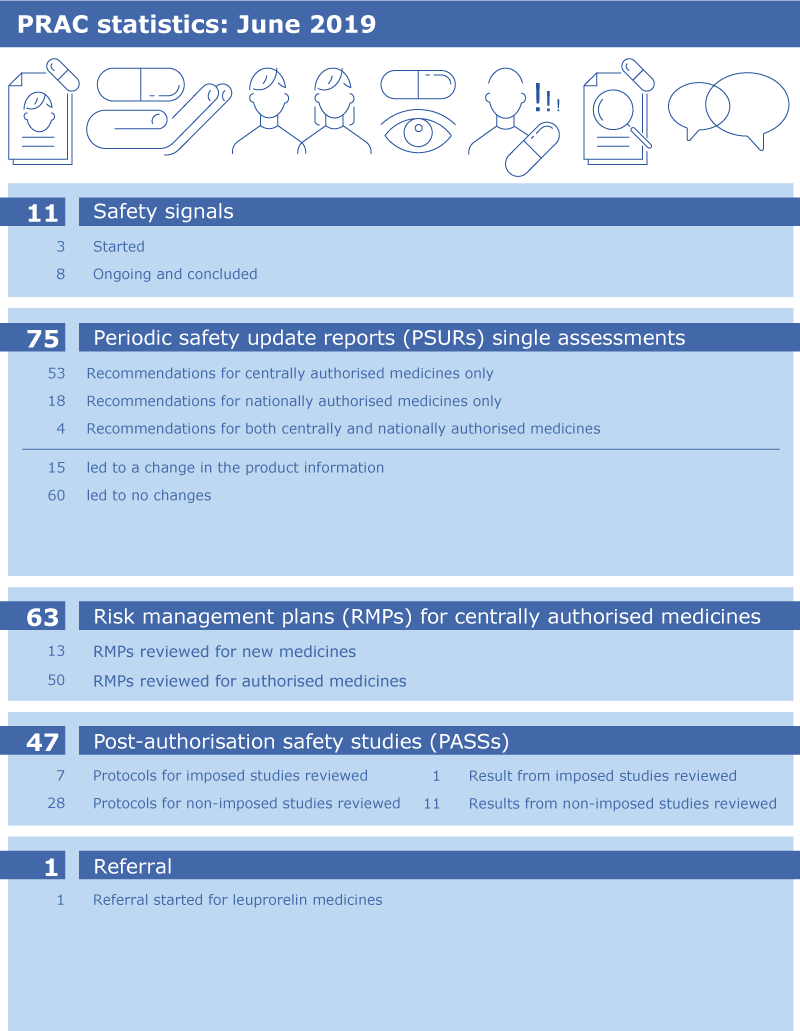
The PRAC’s broad range of responsibilities cover all aspects of the risk management of medicines throughout their lifecycle and include assessment of signals, periodic safety update reports, risk management plans, post-authorisation safety studies and referrals. These terms are explained below:
|
Procedure |
Status |
Update |
|---|---|---|
|
Article-31 procedure: Estradiol-containing (0.01% w/w) medicinal products for topical use |
Under evaluation |
PRAC continued its assessment |
|
Article-31 procedure: Fluorouracil and fluorouracil related substances (capecitabine, tegafur and flucytosine) containing medicinal products |
Under evaluation |
PRAC continued its assessment |
|
Article-20 procedure: Lemtrada |
Under evaluation |
PRAC continued its assessment |
|
Article-31 procedure: Methotrexate containing medicinal products |
Under evaluation |
PRAC continued its assessment |
| Article-20 procedure: Xeljanz | Under evaluation | PRAC continued its assessment |