Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 2-5 September 2019
NewsHumanPharmacovigilanceReferrals
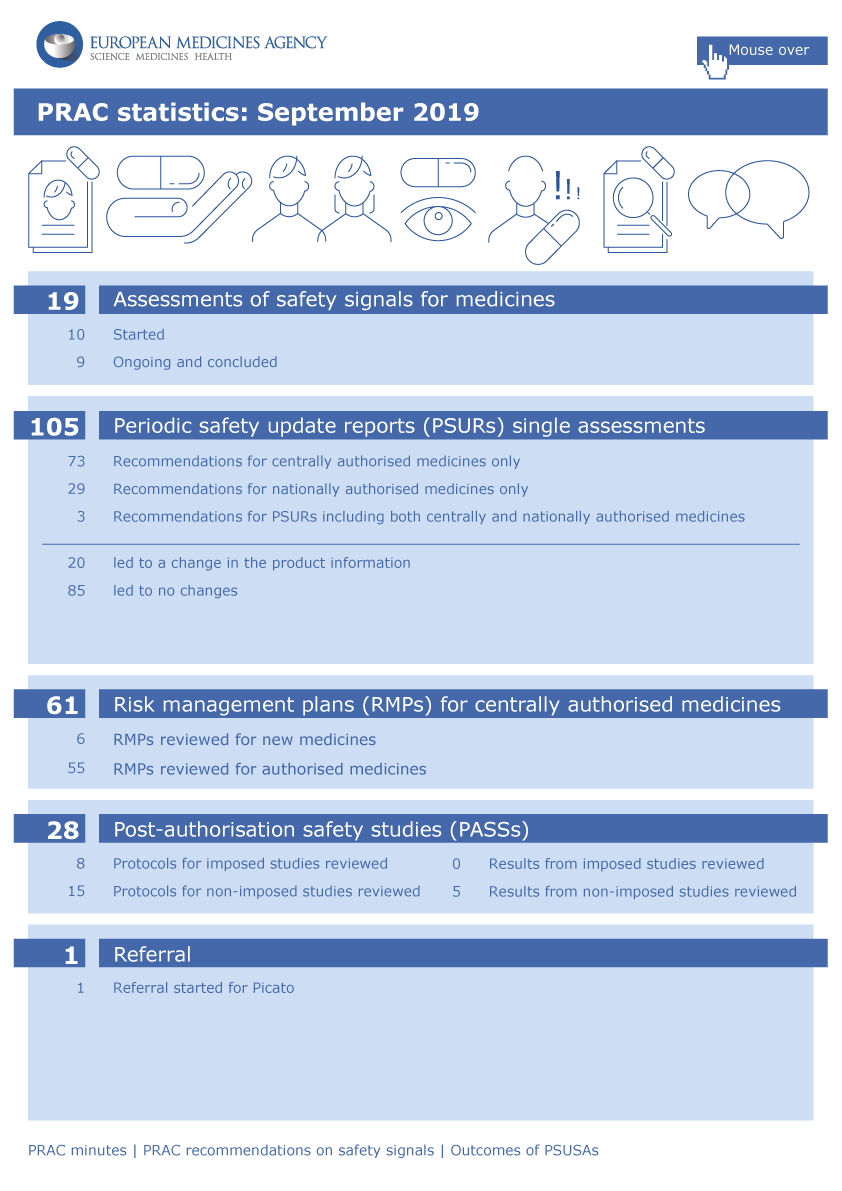
EMA’s safety committee (PRAC) has started a review of data on skin cancer in patients using Picato (ingenol mebutate), a gel for treating actinic keratosis, a skin condition caused by too much sunlight exposure.
The review was triggered by data from several studies showing a higher number of skin cancer cases including cases of squamous cell carcinoma in patients using Picato.
In order to conclude on whether Picato increases the risk of skin cancer, the PRAC will now carry out a thorough review of all available data, including from ongoing studies. The Committee will assess the impact of the data on the benefit-risk balance of Picato and recommend whether the medicine’s marketing authorisation in the EU should be amended.
Healthcare professionals are advised to use Picato with caution in patients who have had skin cancer in the past. In addition, patients should continue to watch out for any skin lesions and inform their doctor immediately if they notice anything unusual.
More information is available below.
Glossary:
Article-20 procedure: Picato
|
Procedure |
Status |
Update |
|---|---|---|
| Article-31 procedure: Cyproterone-containing medicinal products | Under evaluation | PRAC continued its assessment |
|
Article-31 procedure: Estradiol-containing (0.01% w/w) medicinal products for topical use |
Under evaluation |
PRAC adopted a list of experts for the ad-hoc expert group meeting |
|
Article-31 procedure: Fluorouracil and fluorouracil related substances (capecitabine, tegafur and flucytosine) containing medicinal products |
Under evaluation |
PRAC continued its assessment |
|
Article-20 procedure: Lemtrada |
Under evaluation |
PRAC continued its assessment |
|
Article-31 procedure: Leuprorelin-containing depot medicinal products |
Under evaluation |
PRAC continued its assessment |
| Article-20 procedure: Xeljanz | Under evaluation | PRAC adopted a list of outstanding issues for marketing authorisation holders and a list of questions for the ad-hoc expert group |