
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 11-14 November 2024
Leqembi (lecanemab) recommended for treatment of early Alzheimer’s disease
NewsHumanMedicines
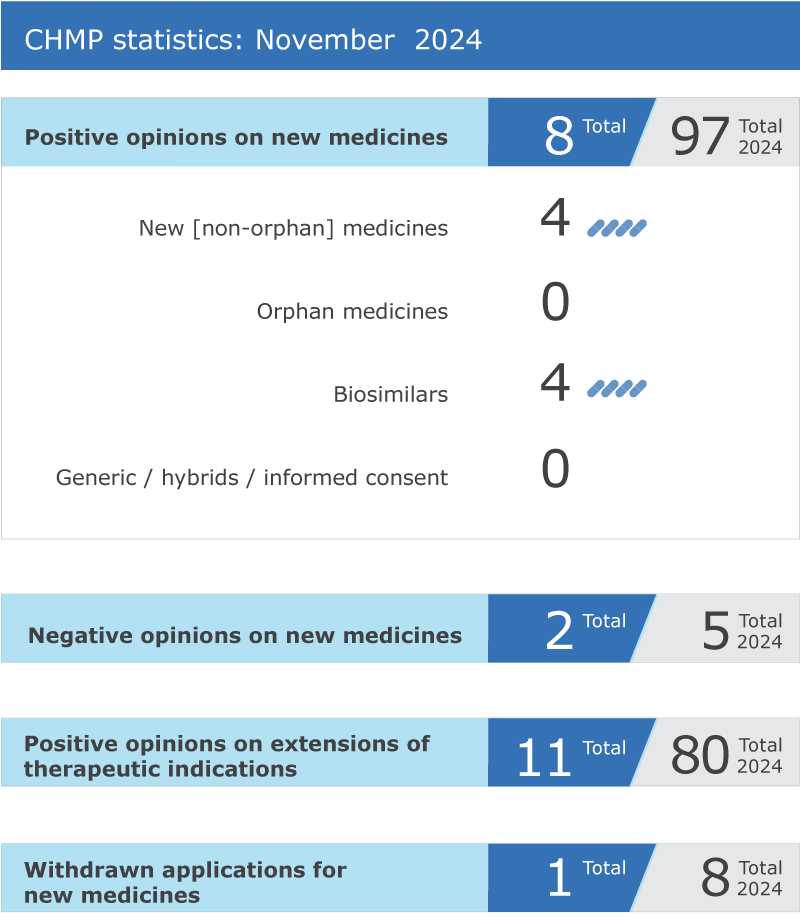
EMA’s human medicines committee (CHMP) recommended eight medicines for approval at its November 2024 meeting.
The committee recommended granting a conditional marketing authorisation for Augtyro (repotrectinib), a medicine intended for the treatment of adults and adolescents with advanced solid tumours, and adults with locally advanced or metastatic non-small cell lung cancer.
The CHMP recommended granting a marketing authorisation under exceptional circumstances for Gohibic (vilobelimab), for the treatment of adult patients with SARS‑CoV2‑induced acute respiratory distress syndrome who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation with or without extracorporeal membrane oxygenation. EMA’s Emergency Task force was consulted during the assessment of this medicine.
Lazcluze (lazertinib) received a positive opinion for the first-line treatment of adult patients with advanced non-small cell lung cancer in combination with amivantamab.
The committee adopted positive opinions for four biosimilar medicines:
The committee recommended extensions of indication for 11 medicines that are already authorised in the EU: CellCept, Evkeeza, Jakavi, Kevzara, Keytruda, Opdivo, Palforzia, Rybrevant, Sarclisa, Tagrisso and Yervoy.
The CHMP recommended the refusal of marketing authorisations for Cinainu (allium/citrus/paullinia/
cacao), a medicine intended for the treatment of moderate-to-severe alopecia areata, a disease causing hair loss of the scalp or other parts of the body, and Kizfizo* (temozolomide), for the treatment of neuroblastoma, a rare cancer that forms from immature nerve cells.
For more information on these negative opinions, see the question-and-answer documents in the grid below.
Following a re-examination, the CHMP has recommended granting a marketing authorisation for Leqembi (lecanemab), for the treatment of mild cognitive impairment (memory and thinking problems) or mild dementia due to Alzheimer’s disease (early Alzheimer’s disease) in patients who have only one or no copy of ApoE4, a certain form of the gene for the protein apolipoprotein E. During the re-examination, the company that applied for authorisation provided additional analyses of the data to support the proposed use in a subgroup of patients. The committee concluded that in the restricted population of patients who have only one or no copy of ApoE4, the benefits of Leqembi outweigh the risks. For more information, see the public health communication in the grid below.
After re-examining its initial opinion, the CHMP recommended updating the advice aimed at minimising the risks of interaction between the weight loss medicine Mysimba (naltrexone/bupropion) and opioid-containing medicines, including painkillers such as morphine and codeine, other opioids used during surgery, and certain medicines for cough, cold or diarrhoea. Opioid medicines may not work effectively in patients taking Mysimba, because one of the active substances in Mysimba, naltrexone, blocks the effects of opioids. There is also a risk of rare but serious and potentially life-threatening reactions, such as seizures and serotonin syndrome (a potentially life-threatening condition that results from having too much serotonin in the body), in people taking Mysimba together with medicines for treating depression and opioids. For more information, see the public health communication in the grid below.
An application for an initial marketing authorisation was withdrawn. Izelvay (avacincaptad pegol) was intended for the treatment of geographic atrophy caused by age-related macular degeneration, a disease that affects the central part of the retina at the back of the eye.
An application to extend the therapeutic indication of Inaqovi (cedazuridine/decitabine) was also withdrawn. It concerned the use in the treatment of acute myeloid leukaemia, to include myelodysplastic syndromes, conditions where the bone marrow does not make enough healthy blood cells or platelets, and chronic myelomonocytic leukaemia, another type of cancer of the white blood cells.
Question-and-answer documents on the withdrawals of these medicines are available in the grid below.
The agenda of the November 2024 CHMP meeting is published on EMA's website. Minutes of the meeting will be published in the coming weeks.
Key figures from the November 2024 CHMP meeting are represented in the graphic below.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
repotrectinib
Bristol-Myers Squibb Pharma EEIG
Treatment of ROS1-positive locally advanced or metastatic non-small cell lung cancer (NSCLC) and for solid tumours
vilobelimab
InflaRx GmbH
Treatment of adult patients with SARS‑CoV2‑induced acute respiratory distress syndrome who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation with or without extracorporeal membrane oxygenation
lazertinib
Janssen Cilag International
Treatment of adult patients with advanced non-small cell lung cancer (NSCLC)
aflibercept
Klinge Biopharma GmbH
Treatment of age-related macular degeneration (AMD) and visual impairment
aflibercept
Formycon AG
Treatment of age-related macular degeneration (AMD) and visual impairment
denosumab
Samsung Bioepis NL B.V.
Treatment of osteoporosis and bone loss
denosumab
Samsung Bioepis NL B.V.
Prevention of skeletal related events with advanced malignancies and treatment of giant cell tumour of bone
mycophenolate mofetil
Roche Registration GmbH
evinacumab
Ultragenyx Germany GmbH
ruxolitinib
Novartis Europharm Limited
sarilumab
Sanofi Winthrop Industrie
pembrolizumab
Merck Sharp & Dohme B.V.
nivolumab
Bristol-Myers Squibb Pharma EEIG
defatted powder of Arachis hypogaea L., semen (peanuts)
Stallergenes
amivantamab
Janssen-Cilag International N.V.
isatuximab
Sanofi Winthrop Industrie
osimertinib
AstraZeneca AB
ipilimumab
Bristol-Myers Squibb Pharma EEIG
liquid ethanolic extract 30 per cent (W/W) of allium cepa fresh bulb and citrus limon fresh fruit / dry aqueous extract of paullinia cupana seed / dry hydroethanolic extract of theobroma cacao seed
Legacy Healthcare (France) S.A.S.
Treatment of alopecia areata in children and adolescents
temozolomide
Orphelia Pharma
Treatment of neuroblastoma
avacincaptad pegol
Astellas Pharma Europe B.V.
decitabine / cedazuridine
Otsuka Pharmaceutical Netherlands B.V.
Inaqovi: withdrawn application (II-0002)
lecanemab
Eisai GmbH
naltrexone hydrochloride / bupropion hydrochloride
Orexigen Therapeutics Ireland Limited