Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 12-15 December 2016
NewsHuman
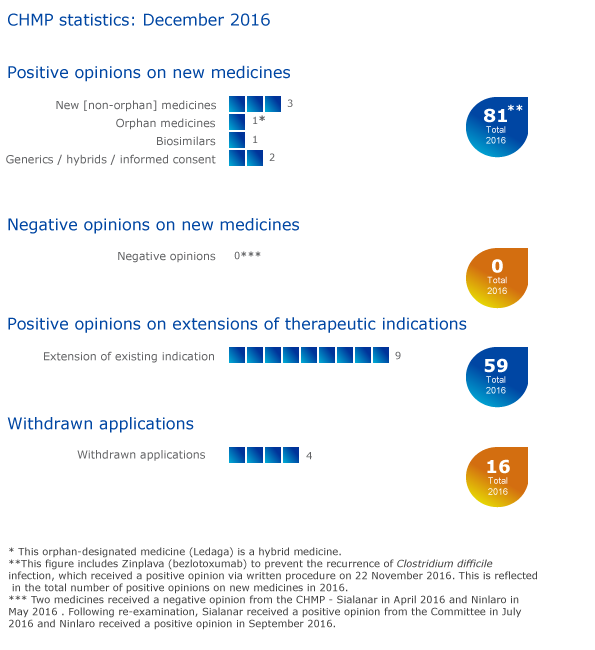
Seven medicines recommended for authorisation, 81 overall in 2016
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended seven new medicines for marketing authorisation at its December 2016 meeting. This brings the total number of medicines recommended for approval by the CHMP in 2016 to 811.
The CHMP recommended granting a marketing authorisation to Olumiant (baricitinib) for the treatment of moderate to severe active rheumatoid arthritis. For more information, please see the press release in the grid below.
The CHMP recommended granting a conditional marketing authorisation for Alecensa (alectinib) for the treatment of anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) previously treated with crizotinib.
Lifmior (etanercept) received a positive recommendation from the Committee for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, axial spondyloarthritis, plaque psoriasis and paediatric plaque psoriasis.
A biosimilar medicine, Truxima (rituximab), received a positive opinion from the CHMP for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukaemia, rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis.
One hybrid medicine, Ledaga (chormethine), received a positive opinion for the treatment of mycosis fungoides-type cutaneous T-cell lymphoma. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data. Ledaga has an orphan designation.
A generic medicine, Pregabalin Zentiva k.s (pregabalin), received a positive opinion from the Committee for the treatment of epilepsy, neuropathic pain and generalised anxiety disorder.
The CHMP also granted a positive opinion for the informed consent application for Vihuma (simoctocog alfa) for the prevention and treatment of bleeding in patients with haemophilia A (congenital factor VIII deficiency). In an informed consent application, reference is made to an authorised medicine and the marketing authorisation holder of the reference medicine has given consent to the use of their dossier in the application procedure.
Positive opinion on Zinplava adopted by written procedure
In addition to the positive opinions for the seven new medicines adopted at the December 2016 meeting, the CHMP recommended granting a marketing authorisation for Zinplava (bezlotoxumab) to prevent the recurrence of Clostridium difficile infection via written procedure on 22 November 2016.
Nine recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Ameluz, Cinryze,Ilaris, Jardiance, Jentadueto, Keytruda, Tivicay, Trajenta and Votubia.
Start of referral: Micro Therapeutics Research Labs, India
The CHMP started a review of medicines for which studies have been conducted by Micro Therapeutic Research Labs at two sites in India. This follows a good clinical practice inspection which raised concerns about the study data used to support marketing authorisation applications of some medicines in the European Union. For more information, please see the start of referral document in the grid below.
Outcome of review of direct-acting antivirals
The CHMP confirmed the recommendation of the Pharmacovigilance Risk Assessment Committee (PRAC) to screen all patients for hepatitis B before starting treatment with direct-acting antivirals for hepatitis C; patients infected with both hepatitis B and C viruses must be monitored and managed according to current clinical guidelines. These measures aim to minimise the risk of hepatitis B re-activation with direct-acting antivirals. For more information, please see the public health communication in the grid below.
Withdrawals of applications
Applications for marketing authorisations for Cavoley (pegfilgrastim), Efgratin (pegfilgrastim), Graspa (eryaspase) and Kepnetic (aceneuramic acid) have been withdrawn. Questions-and-answers documents on these withdrawals are available in the grid below.
A request to extend the indication of Arzerra (ofatumumab) to be used in a new combination with bendamustine for the treatment of relapsed chronic lymphocytic leukaemia has been withdrawn. A questions-and-answers document on this withdrawal is available below.
Agenda and minutes
The agenda of the December 2016 CHMP meeting is published on EMA's website. Minutes of the November 2016 CHMP meeting will be published next week.
CHMP statistics
Key figures from the December 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's December 2016 meeting, is available in the grid below.
More cumulative figures can be found on the medicine evaluation figures page.
1EMA will publish its human medicines highlights for 2016 in early 2017.
| Name of medicine | Alecensa |
|---|---|
| International non-proprietary name (INN) | alectinib |
| Marketing-authorisation applicant | Roche Registration Limited |
| Therapeutic indication | Treatment of anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) previously treated with crizotinib |
| More information | CHMP summary of positive opinion for Alecensa |
| Name of medicine | Lifmior |
|---|---|
| INN | etanercept |
| Marketing-authorisation applicant | Pfizer Limited |
| Therapeutic indication | Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, axial spondyloarthritis, plaque psoriasis and paediatric plaque psoriasis |
| More information |
| Name of medicine | Olumiant |
|---|---|
| INN | baricitinib |
| Marketing-authorisation applicant | Eli Lilly Nederland B.V. |
| Therapeutic indication | Treatment of rheumatoid arthritis |
|
CHMP summary of positive opinion for Olumiant
Press release: New oral treatment for rheumatoid arthritis |
| Name of medicine | Vihuma |
|---|---|
| INN | simoctocog alfa |
| Marketing-authorisation applicant | Octapharma AB |
| Therapeutic indication | Treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency) |
| More information | CHMP summary of positive opinion for Vihuma |
| Name of medicine | Pregabalin Zentiva k.s. |
|---|---|
| INN | pregabalin |
| Marketing-authorisation applicant | Zentiva k.s. |
| Therapeutic indication | Treatment of neuropathic pain, epilepsy and generalised anxiety disorder |
| More information | CHMP summary of positive opinion for Pregabalin Zentiva k.s. |
| Name of medicine | Ledaga |
|---|---|
| INN | chlormethine |
| Marketing-authorisation applicant | Actelion Registration Ltd |
| Therapeutic indication | Treatment of mycosis fungoides-type cutaneous T-cell lymphoma (MF-type CTCL) |
| More information | CHMP summary of positive opinion for Ledaga |
| Name of medicine | Truxima |
|---|---|
| INN | rituximab |
| Marketing-authorisation applicant | Celltrion Healthcare Hungary Kft. |
| Therapeutic indication | Treatment of Non-Hodgkin's lymphoma, chronic lymphocytic leukaemia, rheumatoid arthritis and granulomatosis with polyangiitis and microscopic polyangiitis |
| More information | CHMP summary of positive opinion for Truxima |
| Name of medicine | Ameluz |
|---|---|
| INN | 5-aminolevulinic acid |
| Marketing-authorisation holder | Biofrontera Bioscience GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Ameluz |
| Name of medicine | Cinryze |
|---|---|
| INN | c1-esterase inhibitor, human |
| Marketing-authorisation holder | Shire Services BVBA |
| More information | CHMP post-authorisation summary of positive opinion for Cinryze |
| Name of medicine | Ilaris |
|---|---|
| INN | canakinumab |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Ilaris |
| Name of medicine | Jardiance |
|---|---|
| INN | empagliflozin |
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Jardiance |
| Name of medicine | Jentadueto |
|---|---|
| INN | linagliptin / metformin |
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Jentadueto |
| Name of medicine | Keytruda |
|---|---|
| INN | pembrolizumab |
| Marketing-authorisation holder | Merck Sharp & Dohme Limited |
| More information | CHMP post-authorisation summary of positive opinion for Keytruda |
| Name of medicine | Tivicay |
|---|---|
| INN | dolutegravir |
| Marketing-authorisation holder | ViiV Healthcare UK Limited |
| More information | CHMP post-authorisation summary of positive opinion for Tivicay |
| Name of medicine | Trajenta |
|---|---|
| INN | linagliptin |
| Marketing-authorisation holder | Boehringer Ingelheim International GmbH |
| More information | CHMP Post-authorisation summary of positive opinion for Trajenta (CHMP) |
| Name of medicine | Votubia |
|---|---|
| INN | everolimus |
| Marketing-authorisation holder | Novartis Europharm Ltd |
| More information | CHMP post-authorisation summary of positive opinion for Votubia |
| Name of medicine | Micro Therapeutics Research Labs, India |
|---|---|
| More information | Start of a review concerning the conduct of studies at Micro Therapeutic Research Labs, India |
| Name of medicine | Direct-acting antivirals indicated for the treatment of hepatitis C (interferon free) |
|---|---|
| More information | Direct-acting antivirals for hepatitis C: EMA confirms recommendation to screen for hepatitis B |
| Name of medicine | Lovenox and associated names |
|---|---|
| INN | enoxaparin |
| More information | Questions and answers on Lovenox and associated names (enoxaparin, solution for injection) |
| Name of medicine | Arzerra |
|---|---|
| INN | ofatumumab |
| More information | Questions and answers on the withdrawal of the application for a change to the marketing authorisation for Arzerra (ofatumumab) |
| Name of medicine | Cavoley |
|---|---|
| INN | pegfilgrastim |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Cavoley (pegfilgrastim) |
| Name of medicine | Efgratin |
|---|---|
| INN | pegfilgrastim |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Efgratin (pegfilgrastim) |
| Name of medicine | Graspa |
|---|---|
| INN | eryaspase |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Graspa (eryaspase) |
| Name of medicine | Kepnetic |
|---|---|
| INN | aceneuramic acid |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Kepnetic (aceneuramic acid) |
| Name of medicine | Inovelon |
|---|---|
| INN | rufinamide |
| Marketing-authorisation holder | Eisai Ltd |
| More information | Questions and answers on the outcome of an extension of indication application for Inovelon (rufinamide) |
| Name of medicine | Repatha |
|---|---|
| INN | evolocumab |
| Marketing-authorisation holder | Amgen Europe B.V. |
| More information | CHMP post-authorisation summary of positive opinion for Repatha |