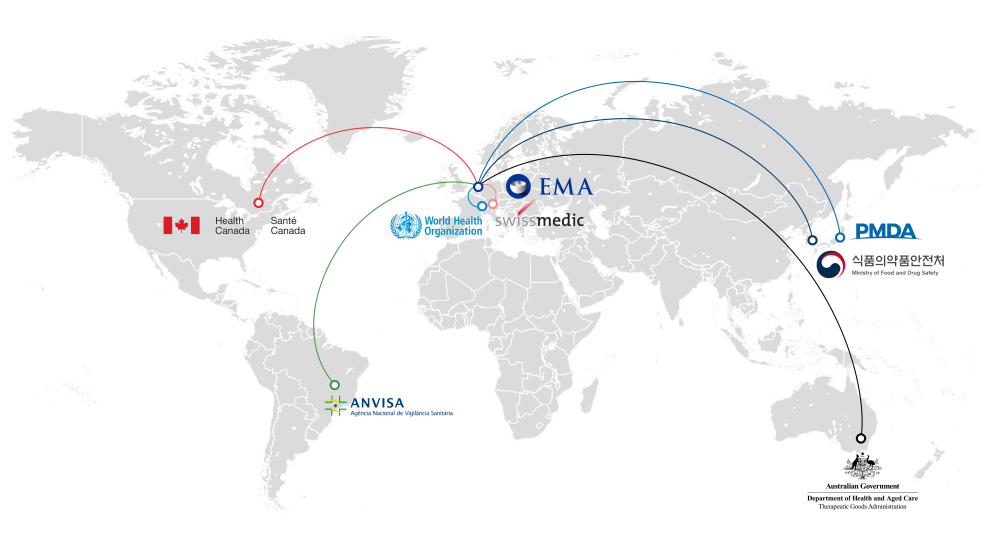
Opening procedures at EMA to non-EU authorities (OPEN) framework
The European Medicines Agency (EMA) collaborates with medicine regulators outside the European Union (EU) in the scientific evaluation of certain medicines, within a framework called OPEN (opening procedures at EMA to non-EU authorities).
CorporateMedicines for use outside the EU