Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 12-15 December 2022
NewsHumanCOVID-19Medicines
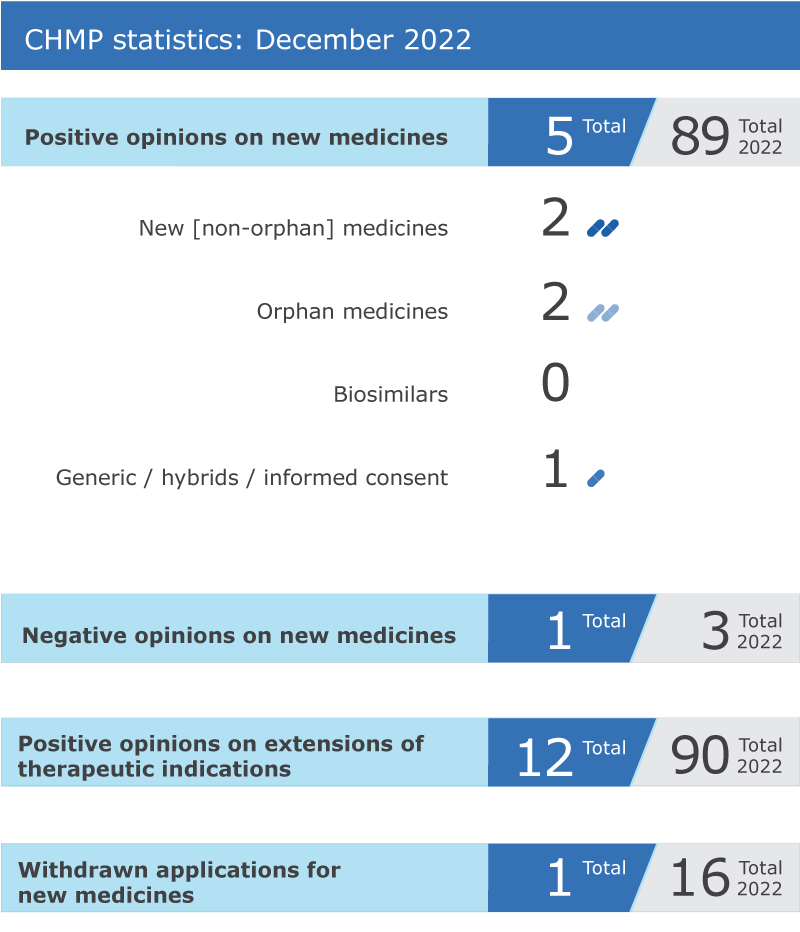
EMA’s human medicines committee (CHMP) recommended five medicines for approval at its December 2022 meeting.
The CHMP recommended granting a conditional marketing authorisation for the advanced therapy medicinal product (ATMP) Hemgenix* (etranacogene dezaparvovec), the first gene therapy for the treatment of severe and moderately severe Haemophilia B. Haemophilia B is an inherited disorder characterised by an increased bleeding tendency due to a partial or complete deficiency in the activity of factor IX, a protein needed to produce blood clots to stop bleeding. Hemgenix was supported through EMA's PRIority MEdicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support to medicines that have a particular potential to address patients' unmet medical needs. See more information in the news announcement in the grid below.
The committee adopted a positive opinion for Imjudo1 (tremelimumab), to be used in combination with Imfinzi (durvalumab) for the treatment of adults with hepatocellular carcinoma, a type of liver cancer.
The committee adopted a positive opinion for Tremelimumab AstraZeneca (tremelimumab), for the treatment of metastatic non-small-cell lung cancer in combination with Imfinzi (durvalumab) and platinum-based chemotherapy.
The CHMP adopted a positive opinion for Pombiliti* (cipaglucosidase alfa) for the treatment of glycogen storage disease type II, also known as Pompe disease. This is a rare, often fatal genetic disorder that causes muscle weakness and disables the heart due to glycogen that builds up in the body cells and nerves.
A generic medicine, Dimethyl fumarate Accord (dimethyl fumarate), received a positive opinion for the treatment of multiple sclerosis, a chronic disease affecting the central nervous system. Dimethyl fumarate Accord is indicated for the treatment of adult and paediatric patients aged 13 years and older.
The CHMP recommended the refusal of a marketing authorisation for Omblastys for the treatment of neuroblastoma, a rare type of cancer. For more information on this negative opinion, see the question-and-answer documents in the grid below.
The committee recommended 12 extensions of indication for medicines that are already authorised in the EU: Adcirca, Dupixent, Edistride, Enhertu, Fintepla, Forxiga, Hemlibra, Imfinzi (includes two new indications), Kerendia, Spikevax and Triumeq.
The application for marketing authorisation for Imbarkyd* was withdrawn. This medicine was intended for the treatment of chronic kidney disease caused by Alport syndrome in adults and children 12 years and above.
The application for extension of therapeutic indication for Olumiant for the treatment of COVID-19 was withdrawn.
Question-and-answer documents on the withdrawals are available in the grid below.
The CHMP completed reviews under Article 29(4), following disagreements among EU Member States regarding two medicines authorisations.
The committee concluded that the benefits of Gelisia outweigh its risks, and the marketing authorisation should be granted in the Netherlands and in the following EU Member States: France, Germany, Italy, Romania and Spain. Gelisia is an eye gel that is used to reduce pressure inside the eye in adults who have ocular hypertension (when the pressure in the eye is higher than normal) or open-angle glaucoma (a disease where the pressure in the eye rises because fluid cannot drain out of the eye).
The committee concluded that the benefits of Rambis outweigh its risks, and the marketing authorisation should be granted in Poland and in the other EU Member States where the company has applied for a marketing authorisation (Czechia and Slovakia). Rambis is a medicine for patients with certain long-term heart conditions and high blood pressure in whom these conditions are well controlled by a combination of two medicines called ramipril and bisoprolol.
For more information about these referrals, see the question-and-answer documents in the grid below.
The committee recommended to extend the use of original Spikevax vaccine and Spikevax bivalent Original/Omicron BA.1 as a booster dose in children aged 6 to 11 years.
The committee recommended converting the conditional marketing authorisation of the COVID-19 vaccine Jcovden to a standard marketing authorisation.
An overview of all the COVID-19 vaccines authorised in the EU is available on EMA’s website.
The agenda of the December 2022 CHMP meeting is published on EMA's website. Minutes of the November 2022 CHMP meeting will be published in the coming weeks.
Key figures from the December 2022 CHMP meeting are represented in the graphic below.
1 The information for Imjudo was corrected on 16 December 2022 to reflect the removal of the orphan designation on 8 December 2022.
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
| Name of medicine | Hemgenix |
| International non-proprietary name (INN) | etranacogene dezaparvovec |
| Marketing-authorisation applicant | CSL Behring GmbH |
| Therapeutic indication | Treatment of severe and moderately severe Haemophilia B |
| More information |
News announcement: First gene therapy to treat haemophilia B |
| Name of medicine | Imjudo |
| INN | tremelimumab |
| Marketing-authorisation applicant | AstraZeneca AB |
| Therapeutic indication | Treatment of hepatocellular carcinoma |
| More information | Imjudo: Pending EC decision |
| Name of medicine | Pombiliti |
| INN | cipaglucosidase alfa |
| Marketing-authorisation applicant | Amicus Therapeutics Europe Limited |
| Therapeutic indication | Treatment of glycogen storage disease type II (Pompe disease) |
| More information | Pombiliti: Pending EC decision |
| Name of medicine | Tremelimumab AstraZeneca |
| INN | tremelimumab |
| Marketing-authorisation applicant | AstraZeneca AB |
| Therapeutic indication | Treatment of metastatic non-small-cell lung cancer |
| More information | Tremelimumab AstraZeneca: Pending EC decision |
| Name of medicine | Dimethyl fumarate Accord |
| INN | dimethyl fumarate |
| Marketing-authorisation holder | Accord Healthcare S.L.U. |
| Therapeutic indication | Treatment of multiple sclerosis |
| More information | Dimethyl fumarate Accord: Pending EC decision |
| Name of medicine | Omblastys |
| INN | iodine (131I) omburtamab |
| Marketing-authorisation holder | Y-Mabs Therapeutics A/S |
| Therapeutic indication | Treatment of neuroblastoma |
| More information | Omblastys: Pending EC decision |
| Name of medicine | Adcirca |
| INN | tadalafil |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | Adcirca: Pending EC decision |
| Name of medicine | Dupixent |
| INN | dupilumab |
| Marketing-authorisation holder | sanofi-aventis groupe |
| More information | Dupixent: Pending EC decision |
| Name of medicine | Edistride |
| INN | dapagliflozin |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Edistride: Pending EC decision |
| Name of medicine | Enhertu |
| INN | trastuzumab deruxtecan |
| Marketing-authorisation holder | Daiichi Sankyo Europe GmbH |
| More information | Enhertu: Pending EC decision |
| Name of medicine | Fintepla |
| INN | fenfluramine |
| Marketing-authorisation holder | Zogenix ROI Limited |
| More information | Fintepla: Pending EC decision |
| Name of medicine | Forxiga |
| INN | dapagliflozin |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Forxiga: Pending EC decision |
| Name of medicine | Hemlibra |
| INN | emicizumab |
| Marketing-authorisation holder | Roche Registration Limited |
| More information | Hemlibra: Pending EC decision |
| Name of medicine | Imfinzi |
| INN | durvalumab |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Imfinzi: Pending EC decision (II-41) |
| Name of medicine | Imfinzi |
| INN | durvalumab |
| Marketing-authorisation holder | AstraZeneca AB |
| More information | Imfinzi: Pending EC decision (II-45) |
| Name of medicine | Kerendia |
| INN | finerenone |
| Marketing-authorisation holder | Bayer AG |
| More information | Kerendia: Pending EC decision |
| Name of medicine | Spikevax |
| INN/common name |
elasomeran / imelasomeran and elasomeran / davesomeran and elasomeran / COVID-19 mRNA vaccine (nucleoside-modified) |
| Marketing-authorisation holder | Moderna Biotech Spain, S.L. |
| More information | Spikevax: Pending EC decision |
| Name of medicine | Triumeq |
| INN | dolutegravir sodium / lamivudine / abacavir (as sulfate) |
| Marketing-authorisation holder | ViiV Healthcare B.V. |
| More information | Triumeq: Pending EC decision |
| Name of medicine | Imbarkyd |
| INN | bardoxolone methyl |
| Marketing-authorisation applicant | Reata Ireland Limited |
| More information | Imbarkyd: Withdrawn application |
| Name of medicine | Olumiant |
| INN | baricitinib |
| Marketing-authorisation holder | Eli Lilly Nederland B.V. |
| More information | Olumiant: Withdrawn application |
| Name of medicine | Gelisia |
| INN | timolol maleate |
| Marketing-authorisation holder | Sifi S.p.A. |
| More information | EMA recommends authorisation of Gelisia (timolol, eye gel) in the EU |
| Name of medicine | Rambis |
| INN | ramipril, bisoprolol fumarate |
| Marketing-authorisation holder | Adamed Pharma S.A. |
| More information | EMA recommends authorisation of Rambis (ramipril / bisoprolol) in the EU |