Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 July 2016
NewsHuman
Eight medicines recommended for approval; use of Truvada extended to include pre-exposure prophylaxis (PrEP) against HIV-1 infection
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended eight medicines for approval at its July meeting.
The CHMP recommended granting marketing authorisations for two medicines for the treatment of advanced renal cell carcinoma (kidney cancer). Cabometyx (cabozantinib) and Kisplyx (lenvatinib) are indicated for the treatment of adult patients with advanced renal cell carcinoma who have been previously treated with a vascular endothelial growth factor (VEGF) inhibitor; Cabometyx is to be used as monotherapy while Kisplyx is for use in combination with everolimus. For more information, please see the press release in the grid below.
The Committee recommended granting a paediatric use marketing authorisation (PUMA) for Sialanar (glycopyrronium bromide) to treat severe drooling in children and adolescents with neurological disorders. This followed a re-examination of the Committee's earlier negative opinion. PUMAs can be granted for medicines which are already authorised, but no longer under patent protection, and that have been developed specifically for children. This is the third time that the Committee has recommended a PUMA since the introduction of this type of marketing authorisation by the Paediatric Regulation, which came into force in 2007. Please see the questions and answers document in the grid below for more information on this opinion.
The Committee recommended granting a marketing authorisation for the orphan medicine Onivyde (irinotecan) for the treatment of metastatic adenocarcinoma of the pancreas.
Truberzi (eluxadoline) was recommended for approval for the treatment of irritable bowel syndrome with diarrhoea.
A generic medicine, Tenofovir disoproxil Zentiva (tenofovir disoproxil), was recommended for approval by the CHMP for the treatment of HIV-1 infection and chronic hepatitis B.
Two biosimilar medicines, Inhixa and Thorinane (both containing enoxaparin sodium), received positive opinions for the prevention and treatment of various disorders related to blood clots in adults.
Five recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Ameluz, Imbruvica, Orencia, Truvada and Xalkori.
A press release on the extension of indication for Truvada as pre-exposure prophylaxis (PrEP) against HIV-1 infection is available in the grid below.
CHMP confirms recommendations for use of Zydelig
The Committee confirmed that the benefits of Zydelig (idelalisib) in the treatment of the blood cancers chronic lymphocytic leukaemia (CLL) and follicular lymphoma outweigh the risk of side effects. However, following a review it has updated recommendations to minimise the risk of serious infections in patients treated with the medicine. For more information, please see the public health communication in the grid below.
CHMP recommends suspension of medicines over flawed studies at Semler Research Centre
The CHMP recommended suspending a number of nationally approved medicines for which bioequivalence studies were conducted at Semler Research Centre Private Ltd, Bangalore, India. For more information, please see the public health communication in the grid below.
Withdrawal of application
The application for a marketing authorisation for Begedina has been withdrawn. A questions and answers document on this withdrawal is available below.
Agenda and minutes
The agenda of the July 2016 meeting is published on EMA's website. Minutes of the June 2016 CHMP meeting will be published next week.
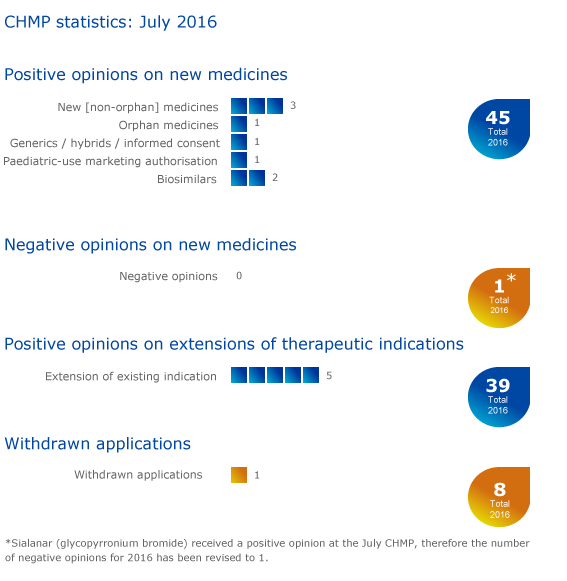
CHMP statistics
Key figures from the July 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP's July 2016 meeting, is available in the grid below.
| Name of medicine | Cabometyx |
|---|---|
| International non-proprietary name (INN) | cabozantinib |
| Marketing-authorisation applicant | Ipsen Pharma |
| Therapeutic indication | Treatment of advanced renal cell carcinoma |
| More information |
CHMP summary of positive opinion for Cabometyx
Press release: Two new medicines for advanced kidney cancer |
| Name of medicine | Onivyde |
|---|---|
| INN | irinotecan |
| Marketing-authorisation applicant | Baxter Innovations GmbH |
| Therapeutic indication | Treatment of metastatic adenocarcinoma of the pancreas |
| More information | CHMP summary of positive opinion for Onivyde |
| Name of medicine | Kisplyx |
|---|---|
| INN | lenvatinib |
| Marketing-authorisation applicant | Eisai Europe Ltd |
| Therapeutic indication | In combination with everolimus for the treatment of unresectable advanced or metastatic renal cell carcinoma |
| More information |
CHMP summary of opinion for Kisplyx
Press release: Two new medicines for advanced kidney cancer |
| Name of medicine | Sialanar |
|---|---|
| INN | glycopyrronium bromide |
| Marketing-authorisation applicant | Proveca Limited |
| Therapeutic indication | Symptomatic treatment of severe sialorrhoea (chronic pathological drooling) in children and adolescents aged 3 years and older with chronic neurological disorders |
| More information |
CHMP summary of opinion for Sialanar
|
| Name of medicine | Truberzi |
|---|---|
| INN | eluxadoline |
| Marketing-authorisation applicant | Aptalis Pharma SAS |
| Therapeutic indication | Treatment of irritable bowel syndrome with diarrhoea |
| More information |
| Name of medicine | Tenofovir disoproxil Zentiva |
|---|---|
| INN | tenofovir disoproxil |
| Marketing-authorisation applicant | Zentiva k.s |
| Therapeutic indication | Treatment of HIV-1 infection and hepatitis B infection |
| More information | CHMP summary of opinion for Tenofovir disoproxil Zentiva |
| Name of medicine | Inhixa |
|---|---|
| International non-proprietary name (INN) | enoxaparin sodium |
| Marketing-authorisation applicant | Techdow Europe AB |
| Therapeutic indication | Prevention and treatment of various disorders related to blood clots in adults |
| More information | CHMP summary of positive opinion for Inhixa |
| Name of medicine | Thorinane |
|---|---|
| International non-proprietary name (INN) | enoxaparin sodium |
| Marketing-authorisation applicant | Pharmathen S.A. |
| Therapeutic indication | Prevention and treatment of various disorders related to blood clots in adults |
| More information |
| Name of medicine | Ameluz |
|---|---|
| INN | 5-aminolevulinic acid |
| Marketing-authorisation holder | Biofrontera Bioscience GmbH |
| More information | CHMP post-authorisation summary of positive opinion for Ameluz |
| Name of medicine | Imbruvica |
|---|---|
| INN | ibrutinib |
| Marketing-authorisation holder | Janssen-Cilag International NV |
| More information | CHMP post-authorisation summary of positive opinion for Imbruvica |
| Name of medicine | Orencia |
|---|---|
| INN | abatacept |
| Marketing-authorisation holder | Bristol-Myers Squibb Pharma EEIG |
| More information | CHMP post-authorisation summary of positive opinion for Orencia |
| Name of medicine | Truvada |
|---|---|
| INN | emtricitabine / tenofovir disoproxil |
| Marketing-authorisation holder | Gilead Sciences International Ltd |
| More information |
CHMP post-authorisation summary of positive opinion for Truvada
Press release: First medicine for HIV pre-exposure prophylaxis recommended for approval in the EU |
| Name of medicine | Xalkori |
|---|---|
| INN | crizotinib |
| Marketing-authorisation holder | Pfizer Limited |
| More information | CHMP post-authorisation summary of positive opinion for Xalkori |
| Name of the procedure | Semler |
|---|---|
| More information | EMA recommends suspension of medicines over flawed studies at Semler Research Centre |
| Name of medicine | Zydelig |
|---|---|
| INN | idelalisib |
| Marketing-authorisation holder | Gilead Sciences International Ltd |
| More information | CHMP confirms recommendations for use of Zydelig |
| Name of medicine | Diclofenac epolamine 50mg tablets |
|---|---|
| INN | diclofenac epolamine |
| Marketing-authorisation holder | Altergon Italia srl |
| More information | Questions and answer on Diclofenac epolamine 50mg tablets |
| Name of medicine | Durogesic and associated names |
|---|---|
| INN | fentanyl |
| Marketing-authorisation holder | Janssen-Cilag group and associated companies |
| More information | Questions and answers on Durogesic and associated names (fentanyl transdermal patches) |
| Name of medicine | Begedina |
|---|---|
| INN | begelomab |
| More information | Questions and answers on the withdrawal of the marketing authorisation application for Begedina (begelomab) |